Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose : SB4 has been approved as a biosimilar of the reference etanercept by the European Commission. Results including one year radiographic progression from the pivotal phase III equivalence study have been previously presented. In this report, the radiographic progression will be further evaluated by different disease activity states in terms of disease activity score by 28 joint count (DAS28) based on erythrocyte sedimentation rate (ESR), simplified disease activity index (SDAI) and clinical disease activity index (CDAI).
Methods : Patients with rheumatoid arthritis (RA) were randomly assigned to receive weekly dose of 50 mg SB4 or ETN for 52 weeks. The proportions of patients achieving remission, low disease activity (LDA), moderate disease activity (MDA), or high disease activity (HDA) in terms of DAS28, SDAI, and CDAI were compared at weeks 12, 24, and 52. Radiographic progression was evaluated using the modified Total Sharp Score (mTSS) at weeks 0 and 52.
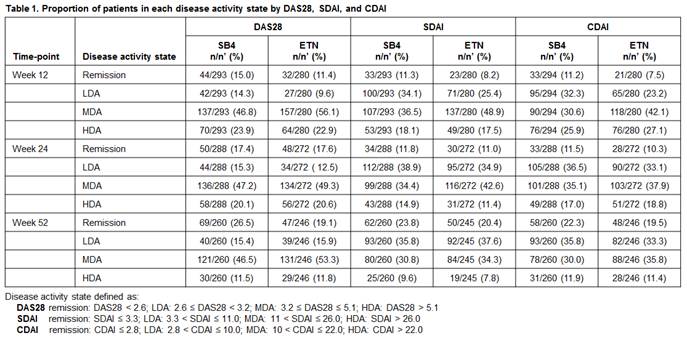
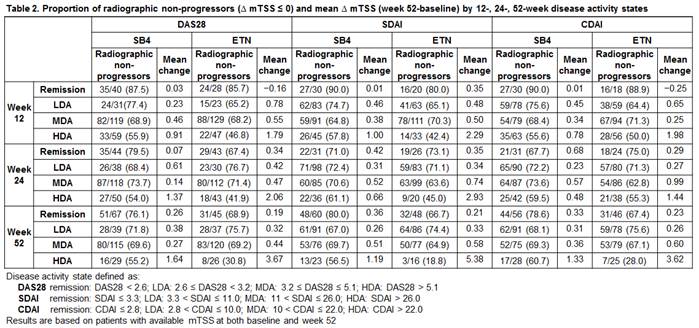
Results: The proportion of patients with remission, LDA, MDA, or HDA was generally comparable between SB4 and ETN at weeks 12, 24, and 52 for different disease activity indices (DAS28, SDAI, CDAI) (Table 1). The proportions of radiographic non-progressors (defined as change in mTSS ¡Â 0) were comparable between SB4 and ETN in each disease activity state with a tendency to decrease as disease activity worsened. In a similar aspect, the radiographic progression evaluated by mTSS was lowest for patients in remission and largest in HDA, and the extent of progression was overall very low in patients with remission, LDA, and MDA (Table 2).
Conclusion : The proportions of patients achieving remission or LDA in the SB4 and ETN treatment groups were comparable at weeks 12, 24, and 52. The radiographic progression was also comparable in each disease activity state with the rate of radiographic non-progressors being highest in patients achieving remission. The overall radiographic progression was very low even in patients with LDA and MDA but slightly higher in HDA.
To cite this abstract in AMA style:
Emery P, Vencovsky J, Ghil J, Cheong SY, Hong E. Evaluation of Radiographic Progression By Disease Activity States in Patients with Rheumatoid Arthritis Treated with SB4 or Reference Etanercept: Results from a Phase III Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/evaluation-of-radiographic-progression-by-disease-activity-states-in-patients-with-rheumatoid-arthritis-treated-with-sb4-or-reference-etanercept-results-from-a-phase-iii-study/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-radiographic-progression-by-disease-activity-states-in-patients-with-rheumatoid-arthritis-treated-with-sb4-or-reference-etanercept-results-from-a-phase-iii-study/