Session Information
Date: Tuesday, November 12, 2019
Title: Epidemiology & Public Health Poster III: OA, Gout, & Other Diseases
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Comparing the outcomes of an intervention in a clinical trial versus an observational study might help identify challenges in translating results from clinical trials to clinical practice. We aimed to describe differences in follow-up data of a clinical trial and an observational study using an empirical example and to evaluate selected methods to account for differences in missing data patterns.
Methods: We used data from two Norwegian rheumatoid arthritis (RA) cohorts, the ARCTIC randomized controlled trial (RCT) and the NOR-VEAC observational study. Both included treatment-naïve patients, implementing treat-to-target strategies. The outcome in the current study was achievement of remission according to the Disease Activity Score in 28 joints at 6, 12 and 24 months. For all analyses, we balanced the cohorts on baseline covariates using inverse probability of treatment weights. As a naïve analysis, we used unadjusted logistic regression in a subset of patients with complete follow-up data. Then, we analyzed the full dataset, combining multiple imputation by chained equations (MICE) to handle missing variables at existing visits and inverse probability of censoring weights (IPCW) to handle missing data due to drop-out. Further, we used three different approaches to handle intermittent missing visits, i.e. missing visits in patients with subsequent study visits: I) Censoring each patient at the first missing visit, II) Ignoring the missing data from single intermittent missing visits and III) Imputing the outcome variables of intermittent missing visits using MICE.
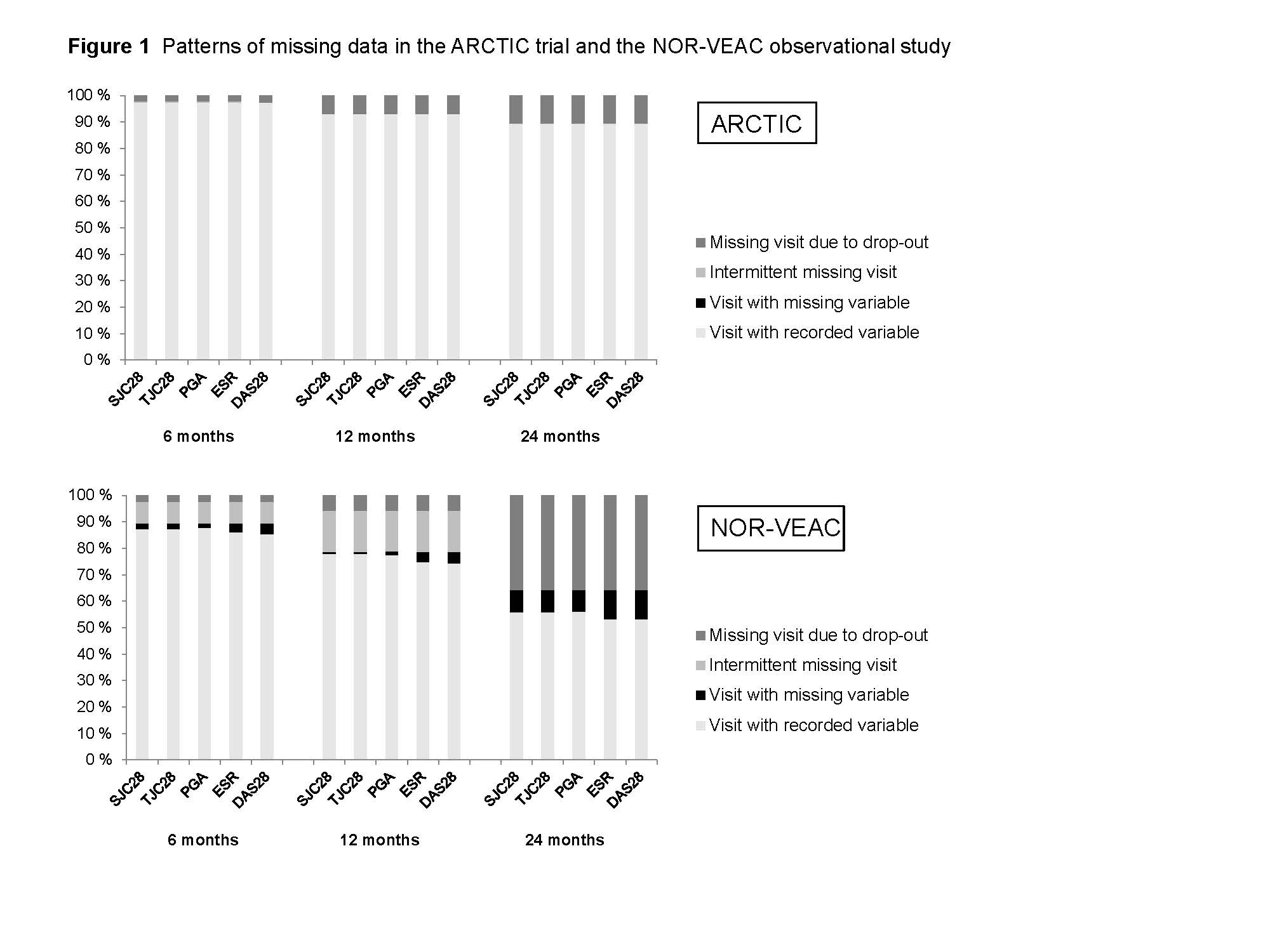
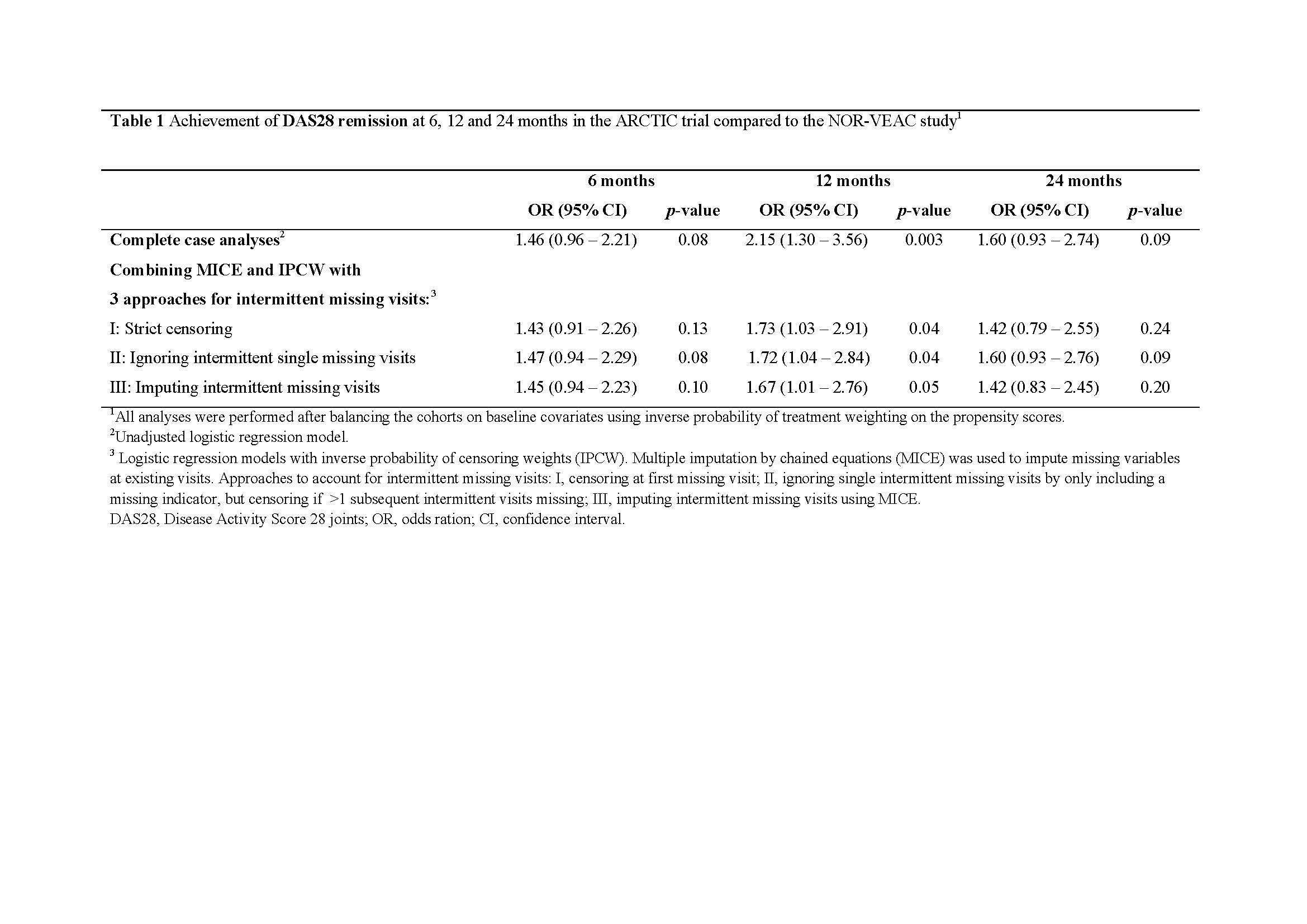
Results: We included 183 patients from the ARCTIC trial and 277 patients from the NOR-VEAC study. During follow-up, missing data in the ARCTIC trial were mainly a result of missing visits due to patients dropping out of the study, whereas missing data in the NOR-VEAC study were a result of missing variables at existing visits, intermittent missing visits or missing visits due to drop-out (Figure 1). The naïve complete case analyses resulted in estimates favoring outcomes in the ARCTIC trial at all study visits during follow-up (Table 1). Combining MICE and IPCW with three different approaches to handle intermittent missing visits yielded similar estimates at 6 months and attenuated estimates at 12 and 24 months (Table 1). At 24 months, odds ratios (ORs) with 95% confidence intervals for reaching remission in the ARCTIC trial vs. the NOR-VEAC study were 1.6 (0.9-2.7) for the complete case analyses and for the combination of MICE and IPCW, 1.4 (0.8-2.6) for the strict censoring approach, 1.6 (0.9-2.8) for the approach ignoring intermittent missing visits and 1.4 (0.8-2.5) (Table 1).
Conclusion: We observed more complex missingness patterns during follow-up in an observational study than in a RCT. The use of methods to account for missing data became more important with longer follow-up time. Compared to complete case analyses, the precision of the effect estimates improved using MICE and IPCW in combination with three different approaches to account for intermittent missing visits. The strict censoring approach might be preferable due to the balance of more precise estimates and simplicity of implementation.
To cite this abstract in AMA style:
Norvang V, Haavardsholm E, Tedeschi S, Lyu H, Kvien T, Mjaavatten M, Solomon D, Yoshida K. Evaluation of Methods to Account for Differential Follow-up When Comparing Clinical Trial Data to Observational Data [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/evaluation-of-methods-to-account-for-differential-follow-up-when-comparing-clinical-trial-data-to-observational-data/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-methods-to-account-for-differential-follow-up-when-comparing-clinical-trial-data-to-observational-data/