Session Information
Date: Tuesday, November 7, 2017
Title: Rheumatoid Arthritis – Clinical Aspects Poster III: Comorbidities
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA). Patients (pts) with RA are at increased risk for herpes zoster (HZ), which is further increased with tofacitinib treatment.1 In a subset of pts in the ORAL Strategy2 randomized controlled trial (RCT), we evaluated the effect of live zoster vaccination (LZV) on HZ rates in methotrexate inadequate responder (MTX-IR) pts with RA who received tofacitinib with or without MTX, or adalimumab (ADA) with MTX.
Methods: ORAL Strategy (NCT02187055) was a Phase 3b/4, 1-year, triple-dummy, active-comparator‑controlled RCT. Pts were randomized 1:1:1 to receive tofacitinib 5 mg twice daily (BID; tofa mono), tofacitinib 5 mg BID + MTX (tofa+MTX), or subcutaneous ADA 40 mg every other week + MTX (ADA+MTX); target MTX dose was 15–25 mg/week. In countries where LZV was available, pts who were ≥50 years old received LZV at the investigator’s discretion, 28 days before the first dose of study drug. HZ incidence rates (IR; pts with events per 100 pt‑years) and 95% confidence intervals (CI) were calculated for each treatment arm and for vaccinated vs non-vaccinated pts.
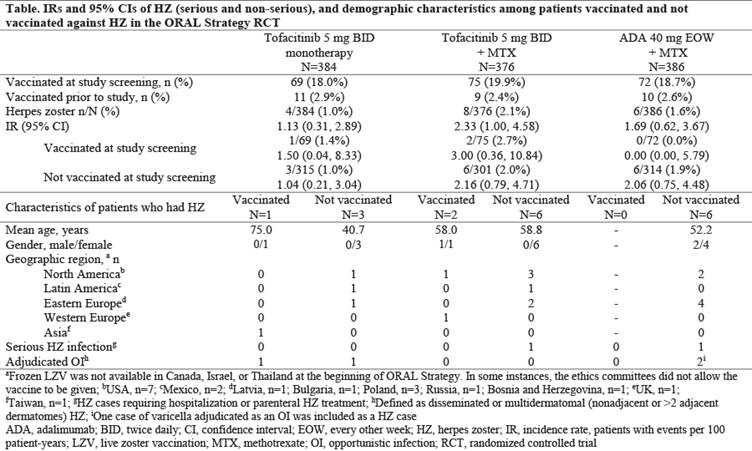
Results: Of 1146 pts who received study drug (mean age: 50.1 years old), 216 received LZV (proportion of pts who received LZV by treatment group: tofa mono: 18.0%; tofa+MTX: 19.9%; ADA+MTX: 18.7%) 28 days before randomization in this RCT; 30 pts self-reported prior vaccination (Table). No pts had zoster-like lesions within 42 days of vaccination; 1 pt had vaccination site erythema. In the overall study population, HZ IR was similar between tofa mono and ADA+MTX, and numerically higher (overlapping CIs) with tofa+MTX. IRs were generally similar for pts who received LZV (18.8% of pts were vaccinated) vs those who did not (81.2%) (Table). Overall, 18/1146 pts had HZ. Among vaccinated pts, 3 (1.4%) had HZ: no events were serious, and 1 (0.5%) event was multidermatomal (with tofa mono). Among pts not vaccinated, 15 (1.6%) had HZ: there were 2 (0.2%) serious HZ events (tofa+MTX: n=1; ADA+MTX: n=1) and 3 (0.3%) multidermatomal events (tofa mono: n=1; ADA+MTX: n=2).
Conclusion: In MTX-IR pts with RA, LZV was well-tolerated. HZ IR was numerically similar between tofa mono and ADA+MTX, and higher with tofa+MTX. HZ rates were generally similar in pts who received LZV vs those not vaccinated. LZV has shown efficacy in prevention of HZ in 51% (pts ≥60 years old) and 70% (50–59 years old) of immunocompetent adults.3 Efficacy of LZV could not be fully evaluated as a minority (<20%) of pts received LZV and not all geographic regions studied in other tofacitinib studies were represented.
References:
- Winthrop KL et al. Arthritis Rheumatol 2014; 66: 2675–84.
- Fleischmann R et al. Lancet 2017 [Epub ahead of print].
- Hales CM et al. MMWR Morb Mortal Wkly Rep 2014; 63: 729–31.
To cite this abstract in AMA style:
Calabrese LH, Abud-Mendoza C, Lindsey S, Lee SH, Takiya L, Iikuni N, Soma K, Luo Z, Fleischmann R. Evaluation of Live Zoster Vaccine in a Subset of Patients with Rheumatoid Arthritis Treated with Tofacitinib with or without Methotrexate, and Adalimumab with Methotrexate: Results from a Phase 3b/4 Randomized Trial [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/evaluation-of-live-zoster-vaccine-in-a-subset-of-patients-with-rheumatoid-arthritis-treated-with-tofacitinib-with-or-without-methotrexate-and-adalimumab-with-methotrexate-results-from-a-phase-3b4-r/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-live-zoster-vaccine-in-a-subset-of-patients-with-rheumatoid-arthritis-treated-with-tofacitinib-with-or-without-methotrexate-and-adalimumab-with-methotrexate-results-from-a-phase-3b4-r/