Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Osteonecrosis of the jaw (ONJ) is a rare but serious adverse event of some antiresorptive therapies, including denosumab (DMAb), and invasive oral procedures and events (OPEs) are suggested to be an important risk factor (Ruggiero, J Oral Maxillofac Surg 2009). The incidence of positively adjudicated ONJ in the DMAb bone loss clinical program is rare (between ≥ 1 and < 10 per 10,000). In this study, we assessed the occurrence of invasive OPEs through Year 5 of the ongoing, 7-year FREEDOM Extension (EXT) trial.
Methods: In FREEDOM, women were randomized to receive DMAb 60 mg SC or placebo every 6 months for 3 years. Those who missed ≤ 1 dose of investigational product and completed the Year-3 visit were eligible for the open-label FREEDOM EXT; women in the EXT long-term group (N = 2343) received DMAb in FREEDOM and EXT, and women in the EXT cross-over group (N = 2207) received placebo in FREEDOM and DMAb in EXT. Women who reached the EXT Year-3 visit were asked to chronicle their history of invasive OPEs (eg, dental implants, tooth extraction, natural tooth loss, or scaling or root planing [extensive subgingival cleaning]) during the EXT. Every 6 months thereafter, women were asked to document their oral health history since the last visit. Jaw surgery information was collected starting from month 30 of the EXT.
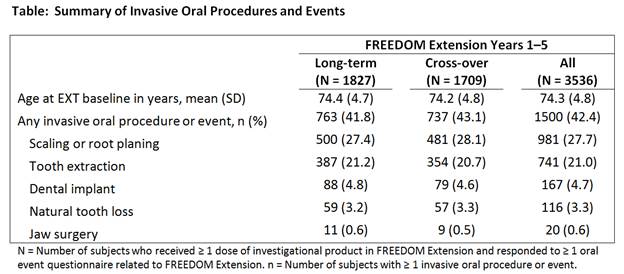
Results: The majority of women (78%) participated in the survey. Over 5 years of the EXT, 42.4% of these women reported an invasive OPE; the incidence of the 5 individual OPEs reported was similar between groups (Table). ONJ incidence was 0.4% (7/1500 subjects) in women reporting invasive OPEs and 0.05% (1/2036 subjects) in women reporting no invasive OPEs. The actual number of invasive OPEs may be underestimated due to limited capture of OPEs in medical charts and due to subjects’ recall bias of events that occurred in the first 3 years of the EXT. During the EXT (Years 1–5), the exposure-adjusted incidence of ONJ was 4.2 per 10,000 patient-years. Of the 8 ONJ cases, 6 have resolved, 1 is ongoing and continues to be followed, and the final outcome of 1 is unknown, as consent was withdrawn.
Conclusion: While invasive OPEs were common in this group of DMAb-treated women with postmenopausal osteoporosis, ONJ incidence was low. Invasive OPEs will continue to be queried prospectively in the EXT to characterize the long-term background rate.
Disclosure:
N. B. Watts,
OsteoDynamics,
1,
AbbVie, Amarin, Amgen Inc., Bristol-Meyers Squibb, Corcept, Endo, Imagepace, Janssen, Lilly, Merck, Novartis, Noven, Pfizer/Wyeth, Radius, Sanofi-aventis,
5,
Merck, NPS,
2,
Amgen Inc., Merck,
9;
J. T. Grbic,
Dentsply Inc. (Dental Implant Division),
2;
M. McClung,
Amgen Inc., Merck,
2,
Amgen Inc., Lilly, Merck,
5;
S. Papapoulos,
Amgen Inc, Merck & Co, Novartis, Axsome, Gador,
5,
Board member International Osteoporosis Foundation,
6,
Amgen Inc., GSK, Novartis, Roche,
8;
D. Kendler,
Astra Zeneca, Eli Lilly, Merck, Novartis, Amgen Inc., Pfizer, Astalis,
2,
Eli Lilly, Merck, Amgen Inc., Pfizer,
5,
Eli Lilly, Amgen Inc., Pfizer, GSK,
8;
C. S. Teglbjaerg,
None;
L. O’Connor,
Amgen Inc.,
1,
Amgen Inc.,
3;
R. B. Wagman,
Amgen Inc.,
1,
Amgen Inc.,
3;
E. Ng,
Amgen Inc.,
1,
Amgen Inc.,
3;
N. S. Daizadeh,
Amgen Inc.,
1,
Amgen Inc.,
3;
P. R. Ho,
Amgen Inc.,
1,
Amgen Inc.,
3.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-invasive-oral-procedures-and-events-in-women-with-postmenopausal-osteoporosis-treated-with-denosumab-results-from-the-pivotal-phase-3-fracture-study-extension/