Session Information
The 2020 Pediatric Rheumatology Symposium, originally scheduled for April 29 – May 2, was postponed due to COVID-19; therefore, abstracts were not presented as scheduled.
Date: Saturday, May 2, 2020
Title: Poster Session 3
Session Type: ACR Abstract Session
Session Time: 4:15PM-5:15PM
Background/Purpose: Rituximab is standard therapy in treating autoimmune brain disease (ABD) including refractory autoimmune encephalitis (AE) and Neuromyelitis Optica Spectrum Disease (NMOSD). Recommendations for continuing concurrent IVIG during rituximab treatment in patients with ABD vary. Mechanisms of rituximab B cell depletion include antibody-dependent cell-mediated cytotoxicity and complement mediated cytotoxicity. In contrast, IVIG’s immunomodulation includes the attenuation of complement-mediated cytotoxicity, blockade of the FC receptors in the reticuloendothelial system, and modulation of NK cells. Despite the frequent use of IVIG as adjunct therapy, there is little data on how concomitant use may affect B-cell depletion and efficacy.
The objective of this study was to determine if concurrent treatment of rituximab with IVIG altered B-cell depletion and time to repopulation in pediatric patients with ABD. We also assessed rates of hypogammaglobinemia and significant infections.
Methods: We conducted a retrospective chart review of 58 patients who received Rituximab for treatment of ABD. There are currently no accepted criteria for a diagnosis of autoimmune encephalitis; however, all patients in this study were determined to meet criteria for probable autoimmune encephalitis by a multidisciplinary team including a pediatric neurologist, rheumatologist and psychiatrist. All patients diagnosed with NMOSD in this study met established criteria. 50 patients met our inclusion criteria of being followed for >1year in our clinic, receiving standard rituximab induction, and having CD19 or CD20 counts recorded following treatment.
We calculated time from the most recent rituximab dosing to repopulation, and noted if IVIG was given within 4 weeks of rituximab dosing. Lymphocyte repopulation was defined as a percentage of CD19 or CD20 cells >0.1. We recorded patients with hypogammaglobinemia and those with significant infections requiring prolonged antibiotic courses or hospitalization.
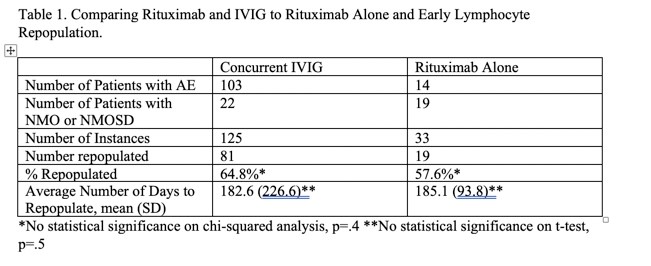
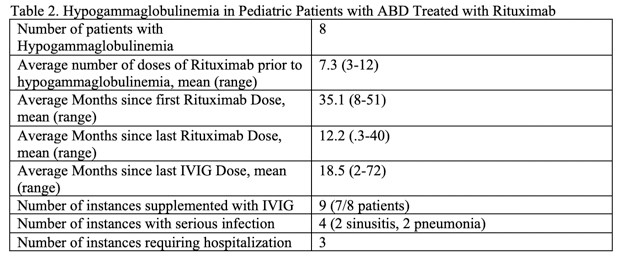
Results: 158 doses of rituximab were given, with 125 given concurrently with IVIG and 33 without. There was no statistical difference in B-cell repopulation in those receiving concurrent treatment, 81 doses(64.8%), compared to rituximab alone, 19 (57.6%). Similarly, there was no statistical difference in mean time to repopulation, with 182.6 days for concomitant treatment and 185.1 days for rituximab alone. Unlike a recent report of high rates of immunodeficiency in autoimmune brain disease, we found hypogammaglobinemia occurred in only 8 of our patients (16% of study population). Within this subset, all were treated for infection related to hypogammaglobinemia except for one, who had persistent low levels without infections. All patients with infection related complications had prompt recovery with replacement dose IVIG and antibiotics. Hypogammaglobinemia was noted on average 12.9 months after last rituximab dose and 18.5 months after last IVIG dose.
Conclusion: There was no difference in rates or time to B cell repletion in patients treated with rituximab alone vs concurrent IVIG treatment. Rates of hypogammaglobinemia were lower in our cohort than recent reports.
To cite this abstract in AMA style:
Wilsey A, Van Mater H, Cannon L. Evaluation of B-cell Depletion with Rituximab and IVIG Concurrent Treatment in Pediatric Autoimmune Brain Disease [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 4). https://acrabstracts.org/abstract/evaluation-of-b-cell-depletion-with-rituximab-and-ivig-concurrent-treatment-in-pediatric-autoimmune-brain-disease/. Accessed .« Back to 2020 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-b-cell-depletion-with-rituximab-and-ivig-concurrent-treatment-in-pediatric-autoimmune-brain-disease/