Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: American rheumatology practice patterns vary considerably. One challenge is practitioner shortage, thus a patient-self-administered screening tool would fill an unmet need if it identified patients with the highest likelihood of having an inflammatory disease (ID).
Objective: To assess the appropriateness of referral patterns and concurrently to evaluate a previously published screening tool, Early Inflammatory Arthritis-3 (EIA–3)1.
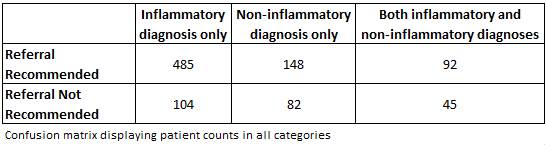
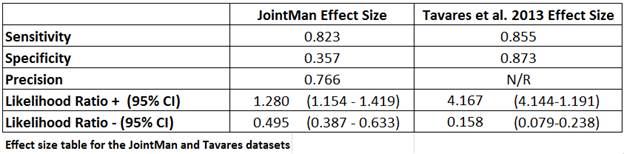
Methods: The US rheumatology database, JointMan® captures inflammatory/non-inflammatory diagnoses of referred patients at a large Washington State rheumatology practice. All patients at the practice fill in an electronic version of the EIA-3 tool. This published screening questionnaire estimates the likelihood of having an inflammatory disease [JIA, RA–fulfilling ACR criteria, Unspecified inflammatory arthritis, SpA, CTDs, SLE] vs non-inflammatory disease [OA, FM, CPPD]. The evaluating clinician was not aware of the EIA-3 results. The results were later correlated to the clinician’s diagnosis. Based on the confusion matrix, effect sizes (sensitivity, specificity and likelihood ratios) were calculated and compared to published results2.
Results: There were 956 patients with a mean age of 55; 66.32% female; 77.82% Caucasian. Of those,726 patients had inflammatory diseases [137 also had non-inflammatory disease]; 230 had non-autoimmune disease. The EIA-3 suggested referrals for 725 patients and no referral for 231.
Conclusion: In this study, primary care physicians adeptly referred appropriate ID patients to less accessible rheumatologists (EIA-3 use only modestly increased preselection of ID patients). It is possible that an electronic patient generated screening tool may help primary providers referring ID patients in less preselected populations (as in the first EIA-3 trial where non-inflammatory diseases were more common).
1. Bell et al. BMC MSK Disorders 2010, 11:50
2. Tavares, R. et al. J. Rheumatol 40(4):417-424
To cite this abstract in AMA style:
Craig G, Knapp K, Ferguson K, Tavares R, Bell M, Schwartzman S. Evaluation of a Pre-Assessment Tool to Define the Spectrum of Autoimmune Diseases in an Underserved Environment [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/evaluation-of-a-pre-assessment-tool-to-define-the-spectrum-of-autoimmune-diseases-in-an-underserved-environment/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-a-pre-assessment-tool-to-define-the-spectrum-of-autoimmune-diseases-in-an-underserved-environment/