Session Information
Session Type: Abstract Submissions
Session Time: 5:30PM-7:00PM
Background/Purpose: Tocilizumab (TCZ) is approved for the treatment of systemic juvenile idiopathic arthritis (sJIA) based on clinical trials in patients ≥2 years of age. This phase 1 study (NP25737), the first of a biologic in patients with sJIA <2 years of age, evaluated the pharmacokinetics (PK), pharmacodynamics (PD), efficacy, and safety of TCZ.
Methods: Patients with uncontrolled sJIA and symptoms for ≥1 month prescreening who failed treatment with corticosteroids and NSAIDs and had no history of allergy to TCZ or other biologics received open-label TCZ 12 mg/kg intravenously (IV) every 2 weeks (dose calculated at each visit based on body weight). Patients were treated up to week 12 and could continue until they reached 2 years of age or were treated for 1 year from baseline. End points included PK (primary) at week 12, PD and efficacy (exploratory), and safety. Comparison was made with exposures from a previous trial in sJIA patients ≥2 years of age (WA18221) that formed the basis for approval of TCZ in sJIA.
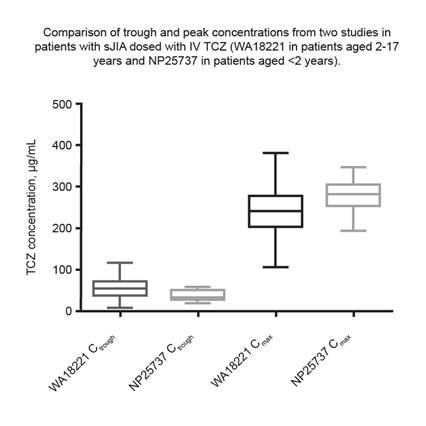
Results: Eleven patients were enrolled; median (range) age was 16 (10-22) months and weight was 10.40 (6.8-11.5) kg. Serum TCZ concentrations, estimated using population PK analysis, peaked immediately after infusion; median (range) maximum concentration was 282 (195-347) μg/mL (steady state reached by week 12) and median (range) trough concentration was 34.3 (19.2-59.7) μg/mL. Peak and trough exposures were within the exposure range in older children (244 [109-382] to 54.3 [10.9-117] μg/mL; Figure). Observed mean±SD soluble IL-6 receptor levels were 47.65±16.40 ng/mL at baseline and 927.83±148.07 ng/mL at day 71. CRP levels were 250.81±425.11 mg/L and 2.80±3.56 mg/L and ESR levels were 59.40±27.47 mm/h and 2.00±1.00 mm/h, respectively. Mean±SD Juvenile Arthritis Disease Activity Score-71 improved from 22.27±10.09 at baseline to 3.66±4.66 at day 71. By week 12, 10 patients had 32 adverse events (AEs); 4 withdrew due to AEs. Infections or infestations were the most frequently reported AEs (10 events, 9 patients). Five serious AEs (SAEs) occurred; 3 patients had SAEs of hypersensitivity that led to treatment withdrawal; 1 of these patients then experienced SAEs of foot and mouth disease and sJIA flare after study withdrawal. No actual cases of MAS were reported, but 2 patients had laboratory abnormalities indicative of MAS according to 2016 criteria (Ravelli A et al. Ann Rheum Dis. 2016;75:481-9). No deaths occurred during the study.
Conclusion: TCZ exposures achieved in this study fell within the exposure range of the previous trial in sJIA patients ≥2 years of age. This study provides evidence that TCZ is effective in sJIA patients <2 years of age and achieves PK and efficacy similar to those demonstrated previously in older patients. The safety profile was similar to that observed in patients ≥2 years of age in types of AEs observed, but there was a higher incidence of serious hypersensitivity events and suspected MAS.
To cite this abstract in AMA style:
Mallalieu NL, Hsu J, Wang K, Wimalasundera S, Wells C, Calvo Penades I, Cuttica RJ, Huppertz HI, Joos R, Kimura Y, Milojevic D, Rosenkranz M, Schikler K, Constantin T, Wouters C. Evaluation of a Dosing Regimen for Tocilizumab in Patients Younger Than Two Years of Age With Systemic Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 4). https://acrabstracts.org/abstract/evaluation-of-a-dosing-regimen-for-tocilizumab-in-patients-younger-than-two-years-of-age-with-systemic-juvenile-idiopathic-arthritis/. Accessed .« Back to 2017 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-a-dosing-regimen-for-tocilizumab-in-patients-younger-than-two-years-of-age-with-systemic-juvenile-idiopathic-arthritis/