Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: GP2015 is an etanercept biosimilar approved for moderate to severely active rheumatoid arthritis (RA), severe active ankylosing spondylitis (AS), and active and progressive psoriatic arthritis (PsA), besides other indications. The COMPACT study is a multicentric, prospective, observational cohort study to evaluate drug persistence, effectiveness, safety and quality of life with GP2015 treatment in patients with rheumatic diseases under real world conditions. Here, we report data form an interim analysis in different patient subgroups with first effectiveness data focusing on the largest indication RA as well as safety data for all enrolled patients.
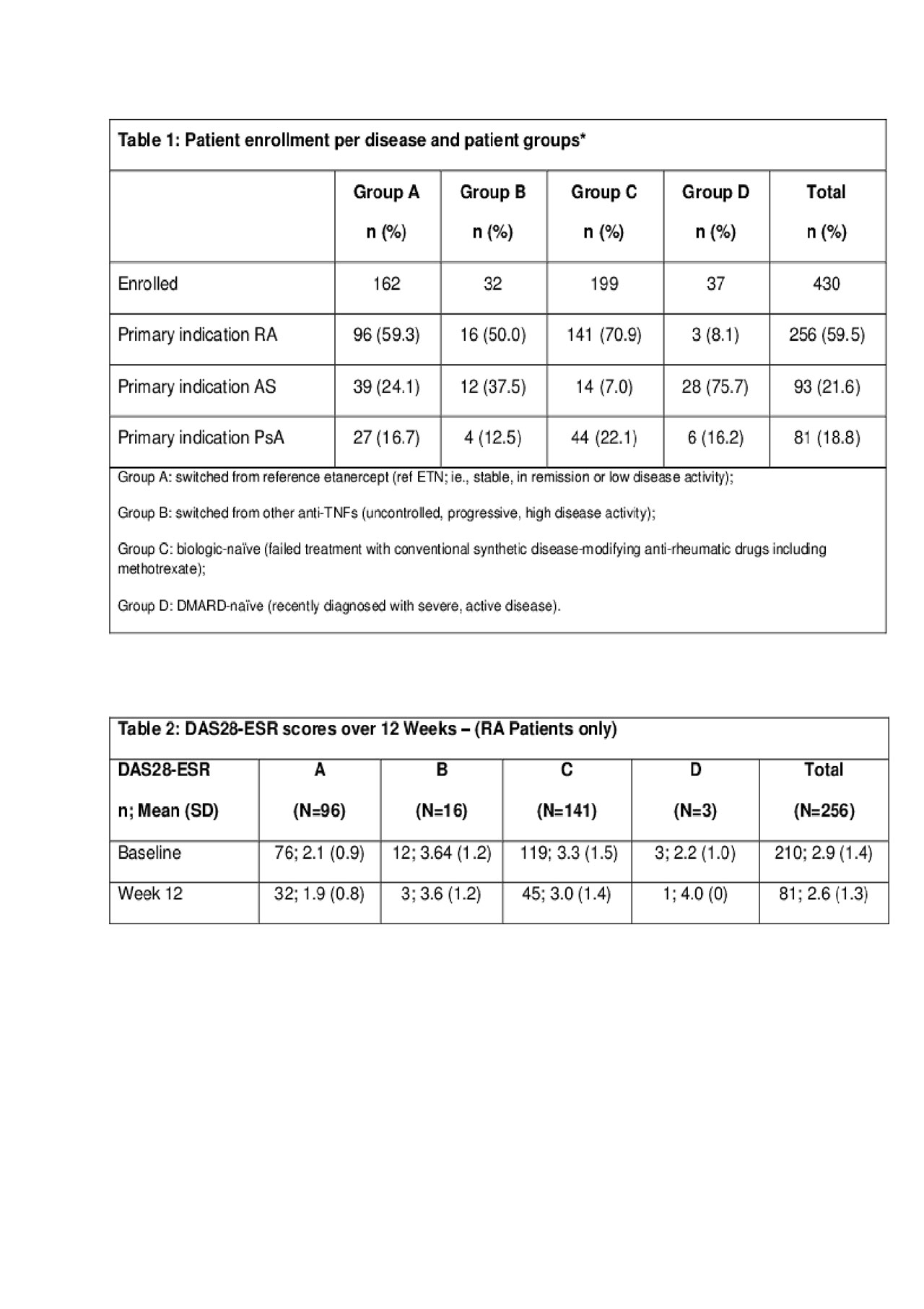
Methods: Patients aged ≥18 years with RA, AS or PsA were initiated treatment with GP2015 prior to enrollment. Patients are categorized based on prior treatment status: (Group A) switched from reference etanercept (ref ETN; ie., stable, in remission or low disease activity); (Group B) switched from other anti-TNFs (previous failure); (Group C) biologic-naïve (failed treatment with conventional synthetic disease-modifying anti-rheumatic drugs including methotrexate (csDMARD–MTX); (Group D) DMARD-naïve (recently diagnosed with severe, active disease). Effectiveness assessments included Disease Activity Score 28-joint count erythrocyte sedimentation rate (DAS28-ESR) until week 12 after enrollment into the study. Functional disability was measured by the Health Assessment Questionnaire Disability Index (HAQ-DI). Incidences of adverse events (AEs), serious AEs (SAE) and adverse drug reactions (ADRs) were reported.
Results: In total, 430 patients were recruited in Germany, UK, Spain, Poland, and Canada until the interim analysis cut-off date in April 2019. Of these, 37.7% of patients were switched from ref ETN, 7.4% were switched from other anti-TNFs, 46.3% were biologic-naïve, and 8.6% were DMARD-naïve. Most patients had RA (59.5%), followed by AS (21.6%) and PsA (18.8%) (Table 1). Comorbidities were more frequent in RA patients (77.7%) than PsA (66.7%) or AS (57%) patients and systemic corticosteroids use was highest in RA patients (47.7%). For RA patients as the largest population, total mean DAS28-ESR scores were 2.9 (1.4) for all 4 groups at baseline, and decreased to 2.6 (1.3) by week 12 (Table 2). HAQ-DI scores in RA patients changed from 0.88 to 0.76 until week 12. Overall, 33% of the patients had at least one AE, while 2.8% of the patients discontinued due to AEs and 8.4% required interruption of the study drug due to AEs (Table 3). No deaths occurred in the study.
Conclusion: COMPACT study is an ongoing observational study of treatment of RA/PsA/AS patients with GP2015 etanercept biosimilar. This interim analysis on real-world treatment with GP2015 showed initial results of effectiveness and safety. Patient distribution in the different patient groups reflect real-life situation. Comorbidities in RA patients were more frequently reported than in AS and PsA. No new safety signals were observed compared to previously published data on etanercept1-3.
References:
- Davis JC, et al. Ann Rheum Dis 2008;67:346-352.
- Weinblatt ME, et al. Arthritis Care Res (Hoboken). 2011:Mar;63(3):373-82.
- Matucci-Cerinic M, et al. RMD Open. 2018 Nov 14;4(2):e000757.
To cite this abstract in AMA style:
Schmalzing M, Askari A, Walsh D, Castro M, De Toro F, Jeka S, Kellner H, Friccius-Quecke H, Furlan F, Hachaichi S, Sheeran T. Etanercept Biosimilar GP 2015 (Erelzi®) in Rheumatic Diseases: Interim Analysis of Real-World Data from COMPACT: A Multicentric, Prospective, Observational Cohort Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/etanercept-biosimilar-gp-2015-erelzi-in-rheumatic-diseases-interim-analysis-of-real-world-data-from-compact-a-multicentric-prospective-observational-cohort-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/etanercept-biosimilar-gp-2015-erelzi-in-rheumatic-diseases-interim-analysis-of-real-world-data-from-compact-a-multicentric-prospective-observational-cohort-study/