Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Epratuzumab, a monoclonal antibody targeting CD22, is in development for the treatment of systemic lupus erythematosus (SLE). Two randomized controlled trials (RCTs): ALLEVIATE 1 and 2 were prematurely terminated because of interruption of drug supply. SL0006 was an open-label extension study in which patients previously enrolled in the ALLEVIATE trials received epratuzumab.1 Assessment of health-related quality of life (HRQoL) alongside measures of disease activity and damage is recommended in SLE RCTs and provides a comprehensive view of therapeutic responses.2,3 This presentation reports final data from SL0006 on the effect of epratuzumab on HRQoL measured using the 36-item Short Form Health Survey (SF-36).
Methods:
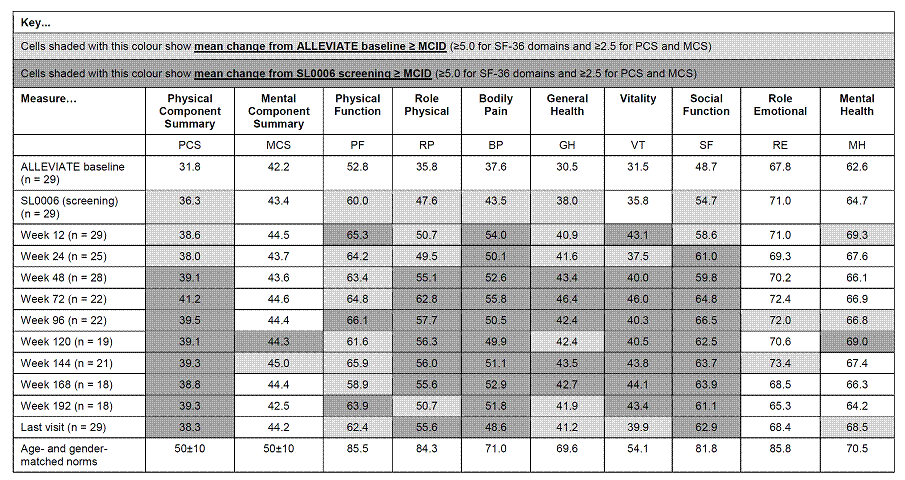
All patients enrolled in ALLEVIATE US sites who received randomized treatment (n = 60) and satisfied all inclusion criteria were eligible for enrollment in SL0006. Twenty-nine patients (90% female, 79% Caucasian, mean age 40 years) entered SL0006, having received placebo (n = 8), 360 (n = 17) or 720 (n = 4) mg/m2 epratuzumab during the ALLEVIATE double-blind trials. In SL0006, all patients received 12-week cycles of 360 mg/m2 epratuzumab; two infusions on days 1 and 8 of each cycle. SF-36 was assessed at screening and every 4 weeks thereafter. Mean changes were compared to ALLEVIATE baseline and SL0006 screening values.
Results:
The table shows means and mean changes in PCS and MCS and individual SF-36 domain scores vs age- and gender-matched norms.4 At ALLEVIATE baseline, PCS and MCS scores were approximately 2 and 1 standard deviations below normative values, respectively; domain scores 7.9–48.5 points lower than age- and gender-matched norms, reflecting the broad impact of active SLE on HRQoL. At the SL0006 screening visit, mean changes from ALLEVIATE baseline (BL) in SF-36 PCS and 5 domain scores met or exceeded minimal clinically important differences (MCID). Throughout SL0006, these improvements were maintained or improved up to and including week 192, after which patients numbers were <50% of the screening population. Changes from ALLEVIATE baseline scores were ≥ MCID in PCS and all its domains at all timepoints, apart from RE and MH, where numerical improvements were evident and ≥ MCID at some visits. Further improvement during SL0006 was evidenced by clinically meaningful changes from the screening visit at most timepoints in PCS, RP, BP, GH, VT and SF, and at the last available visit for PCS RP, BP and SF.
Conclusion:
In an open-label, single-arm extension to 2 double-blind studies, patients receiving epratuzumab reported clinically meaningful improvements in HRQoL that were sustained over approximately 4 years of treatment.
References
1. Strand. Arthritis Rheum 2008;57(Suppl):1086.
2. Jolly. Curr Rheumatol Rep 2010;12:229-36.
3. Strand et al. Expert Rev Pharmacoecon Outcomes Res 2011;11:455-68.
4. Strand et al. Ann Rheum Dis 2007;66:482.
Disclosure:
V. Strand,
UCB Pharma,
5;
K. Hobbs,
HGS, UCB Pharma,
5;
D. J. Wallace,
BMS, Genentech and Biogen IDEC Inc, Humen Genome Sciences Inc, MedImmune, Novo Nordisk and UCB Pharma,
5;
K. Kalunian,
Bristol-Myers Squibb, Genentech and Biogen IDEC Inc, Anthera, MedImmune, Novo Nordisk, Zymogenetics, Serono and UCB Pharma,
5,
Genentech and Biogen IDEC Inc, Cephalon, Cypress, MedImmune, Novo Nordisk and UCB Pharma,
2;
B. Kilgallen,
UCB Pharma,
3;
E. Nikaï,
UCB Pharma,
3;
W. A. Wegener,
Immunomedics, Inc.,
1,
Immunomedics, Inc.,
3;
D. M. Goldenberg,
Immunomedics, Inc.,
3,
Immunomedics, Inc.,
1,
Immunomedics, Inc.,
6.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/epratuzumab-treated-systemic-lupus-erythematosus-patients-report-improvements-in-health-related-quality-of-life-final-results-from-an-open-label-extension-study-sl0006/