Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Epratuzumab is a monoclonal antibody targeting CD22. In EMBLEMTM (dose-ranging phase IIb study), epratuzumab produced clinically relevant improvements in disease activity in patients with moderate-to-severe systemic lupus erythematosus (SLE).1 The open-label extension (OLE) of EMBLEMTM (NCT00660881) reports long-term data on the efficacy of epratuzumab.
Methods:
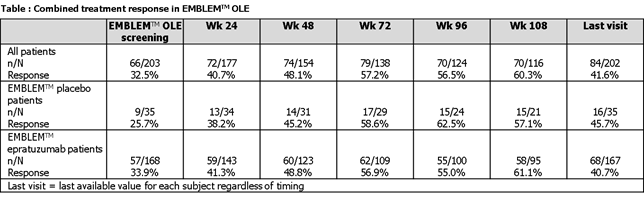
Patients from any EMBLEMTM arm completing 12 weeks blinded treatment and those who discontinued due to lack of efficacy but completed ≥8 weeks were eligible. In the OLE, all patients received 1200mg epratuzumab at weeks 0 and 2 of repeating 12-week cycles. Evaluation visits were at weeks 4 and 8 of each cycle. Efficacy endpoints included British Isles Lupus Assessment Group (BILAG) improvement, SLE Disease Activity Index (SLEDAI) score, Physician Global Assessment (PGA) and combined treatment response (defined as BILAG improvement without worsening, no SLEDAI worsening, no PGA worsening, relative to EMBLEMTM baseline [BL]). Observed data are reported to week 108 of OLE (last timepoint when >50% of patients reported data for BILAG). The last visit, which refers to the last available value for each subject regardless of timing, is also presented.
Results:
203 patients participated in the EMBLEMTM OLE: 95% female (n=192), 78% Caucasian (n=158), mean±SD age 39±11 yrs. 35 (17%) and 168 (83%) received placebo and epratuzumab (various doses) respectively for 12 weeks in EMBLEMTM. Median (range) duration of epratuzumab exposure was 845 (75–1185) days. BILAG improvements were observed between EMBLEMTM BL and week 108 of OLE. Median BILAG total score decreased by 64%. Median (range) BILAG total score was 25.0 (12–61) at EMBLEMTM BL, 14.0 (0–57) at OLE screening, 9.0 (0–52) at week 48, 9.0 (0–52) at week 96 and 9.0 (0–52) at week 108. Last visit value was 10.0 (0-72). At week 108, 60.3% of on-going patients responded to treatment, according to combined treatment response criteria (Table). Median (range) SLEDAI total score was 12.0 (6–39) at EMBLEMTM BL, 10.0 (0–34) at OLE screening, 6.0 (0–30) at week 48, 5.0 (0–22) at week 96, 4.0 (0–24) at week 108 and last visit value was 8.0 (0-32). At week 108, 94.0% (109/116) had no worsening in SLEDAI. Median (range) PGA total score was 50.0 (9–90) at EMBLEMTM BL, 31.0 (0–96) at OLE screening, 18.0 (0–81) at week 48, 19.0 (0–73) at week 96, 17.5 (0–69) at week 108 and last visit value was 25.0 (0-94). At week 108, 97.4% (113/116) had no worsening in PGA. Corticosteroid use decreased with long-term epratuzumab use.
Conclusion:
Epratuzumab was associated with sustained improvements in disease activity in patients with moderate-to-severe SLE. Responder rates were sustained beyond 2 years or increased during open-label treatment, particularly in patients previously treated with placebo.
References:
1. Wallace DJ, et al. Ann Rheum Dis, Online First 12 January 2013. annrheumdis-2012-202760.
Disclosure:
M. E. B. Clowse,
UCB Pharma,
5;
F. Houssiau,
UCB Pharma,
2,
UCB Pharma,
5;
M. A. Petri,
UCB Pharma,
2,
UCB Pharma,
5;
B. Kilgallen,
UCB Pharma,
1,
UCB Pharma,
3;
K. Kalunian,
Genentech, Biogen IDEC Inc, Cephalon, Cypress, MedImmune, Novo Nordisk, UCB Pharma,
2,
Bristol-Myers Squibb, Genentech, Biogen IDEC Inc, Anthera, MedImmune, Novo Nordisk, Zymogenetics, Serono, UCB Pharma,
5;
V. Strand,
Abbott Immunology Pharmaceuticals, Amgen Inc, AstraZeneca, Biogen Idec, Canfite Pharma, Centocor Inc, Cypress Biosciences Inc, Euro-Diagnostica Inc, Fibrogen, Forest Laboratories, Genentech, Human Genome Sciences Inc, Incyte, Novartis Pharmaceuticals Corp,
5;
S. Bongardt,
UCB Pharma,
3;
C. Gordon,
GSK, MedImmune, Merck Serono, Parexel and UCB Pharma ,
5;
D. J. Wallace,
Bristol-Myers Squibb, Genentech, Biogen IDEC Inc, GlaxoSmithKline, Human Genome Sciences Inc, MedImmune, Novo Nordisk and UCB Pharma,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/epratuzumab-maintains-improvements-in-disease-activity-for-over-2-years-in-patients-with-moderate-to-severe-systemic-lupus-erythematosus-results-from-an-open-label-long-term-extension-study/