Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Enthesitis, a symptom commonly associated with psoriatic arthritis (PsA), negatively affects quality of life. Although enthesitis is routinely reported in randomized clinical trials of new therapies for PsA, it has not been well characterized in real-world patient populations. The aim of this study is to determine the frequency and severity of enthesitis and describe treatment patterns in PsA patients with enthesitis in Europe and Japan.
Methods: Data were analysed from the Adelphi PsA Disease Specific Programme, a cross-sectional survey of 344 rheumatologists (and orthopedic surgeons and internists in Japan), 171 dermatologists and their consulting patients in France, Germany, Italy, Spain, UK (5EU) and Japan from 03/2018 to 11/2018. Participating physicians completed patient record forms capturing clinical and treatment information based on medical record data. Patients with a physician-confirmed diagnosis of PsA who were ≥18 years old and not participating in a clinical trial were eligible for inclusion. The proportion of patients with enthesitis was assessed overall and by country at several times during the PsA disease course. For patients currently experiencing enthesitis, data describing enthesitis severity and PsA-related treatments were collected.
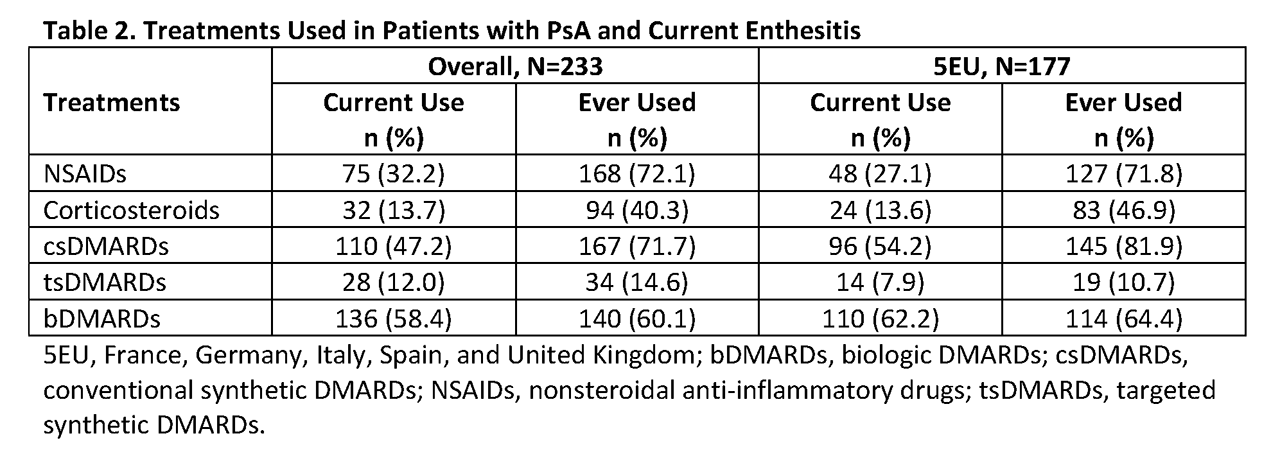
Results: 1945 patients with PsA were included. Mean age was 48.9 (SD 13.6) years, 54.2% were male, and mean time since diagnosis was 59.3 (SD 5.9) months (n=1595). Overall, 26.8% of patients ever had enthesitis, 12.0% of patients currently had enthesitis, 22.0% had enthesitis at the time of PsA diagnosis, and 23.8% had enthesitis at their most recent PsA-related treatment initiation (Table 1). At each time point assessed, the proportion of patients with enthesitis was greatest in Japan and lowest in Germany. Of patients with current enthesitis, 28.8% of cases were moderate or severe compared with 70.0% of cases at diagnosis and 61.8% of cases at initiation of patients’ most recent PsA-related treatment regimen. The most frequently used medications among patient with current enthesitis were biologic DMARDs (58.4%), conventional synthetic DMARDs (47.2%), and NSAIDs (32.2%) (Table 2).
Conclusion: Enthesitis is a common disease manifestation in real-world PsA patients in Europe and Japan. The majority of patients with current enthesitis were treated with advanced therapies; physicians rated patients’ enthesitis as moderate or severe in 28.8% of those who continued to have enthesitis despite treatment, suggesting an unmet need and target for future PsA therapies.
Medical writing services provided by Joann Hettasch (Fishawack Group, US) and funded by AbbVie.
To cite this abstract in AMA style:
Aletaha D, Zueger P, Chen N, Booth N. Enthesitis Frequency and Treatment Patterns in Patients with Psoriatic Arthritis in Europe and Japan [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/enthesitis-frequency-and-treatment-patterns-in-patients-with-psoriatic-arthritis-in-europe-and-japan/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/enthesitis-frequency-and-treatment-patterns-in-patients-with-psoriatic-arthritis-in-europe-and-japan/