Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The clinical Disease Activity in Psoriatic Arthritis (cDAPSA) score is widely used to assess PsA disease activity and its trajectory. cDAPSA sums four measures: 2 patient-reported outcomes (PROs), pain and patient global visual analog scales, and 66 swollen/68 tender joint counts (66SJC, 68TJC) into a single score despite their different scales. It therefore may obscure clinically important patterns, especially when inflammation and symptoms are discordant. To address this, we (1) analyzed the contributions of individual cDAPSA components, (2) developed a two-score measure (cDAPSA2), and (3) evaluated its utility in predicting patient outcomes.
Methods: To perform Principal Components Analysis (PCA), we obtained cDAPSA-adjusted residuals for pain, patient global, 66SJC, and 68TJC at baseline. These residuals were generated by regressing each variable on a smooth function of cDAPSA to account for nonlinear associations; they collectively represent available information about the patient’s disease state and trajectory over and above what is described by cDAPSA. PCA was then applied to the residuals to find a single measure (the dominant principal component) that explains the largest fraction of remaining variation. To assess whether this first PC can risk-stratify patients, we modeled longitudinal cDAPSA trajectories using linear mixed-effects models with a smooth function of time for each quartile of the 1st PC, adjusting for age, sex, race, BMI. We further fit a Cox proportional hazard model to study the associations between PC quartiles and time to remission (cDAPSA ≤ 4), adjusting for age, sex, BMI, and baseline cDAPSA.
Results: Among 752 PsA patients fulfilling the PsA CASPAR classification (median age 52.8 years; BMI 29.4 kg/m²; follow-up 2.0 years), PCA identified what we call the PROs-Joint Contrast (PJC), that is, approximately the mean PRO scores minus the mean joint count scores. For a given cDAPSA level, PJC measures whether most of the cDAPSA is due to the PRO components or to the joint counts. PJC accounts for 49.5% of the residual variance beyond what can be described by cDAPSA itself (Table 1). All PROMIS Profile measures, enthesitis, and sex significantly differed across the PJC quartiles (Table 2).Longitudinal analysis showed the 1st and 4th quartiles of the PJC start at the same level, higher than Q2/Q3 and that Q1 has a steeper decline while Q4 remains at higher levels over a 5-year follow-up (Figure 1). Remission rates were statistically significantly associated with PJC, (HR per 1 SD: 0.88; 95% CI: 0.77–0.99). The 4th quartile had a significantly lower remission rate than the 1st quartile (HR: 0.49; 95% CI: 0.27–0.88).
Conclusion: Based on these findings, we propose cDAPSA2 as a two-score measure combining traditional cDAPSA with PJC. For example, patients with high cDAPSA and low PJC may benefit from escalating DMARDs targeting inflammation, while those with similar cDAPSA and high PJC may require symptom-focused management and additional diagnostics before altering DMARD therapy. cDAPSA2 captures both overall severity and underlying heterogeneity of PsA, supporting a more nuanced assessment of PsA activity and individualized treatment.
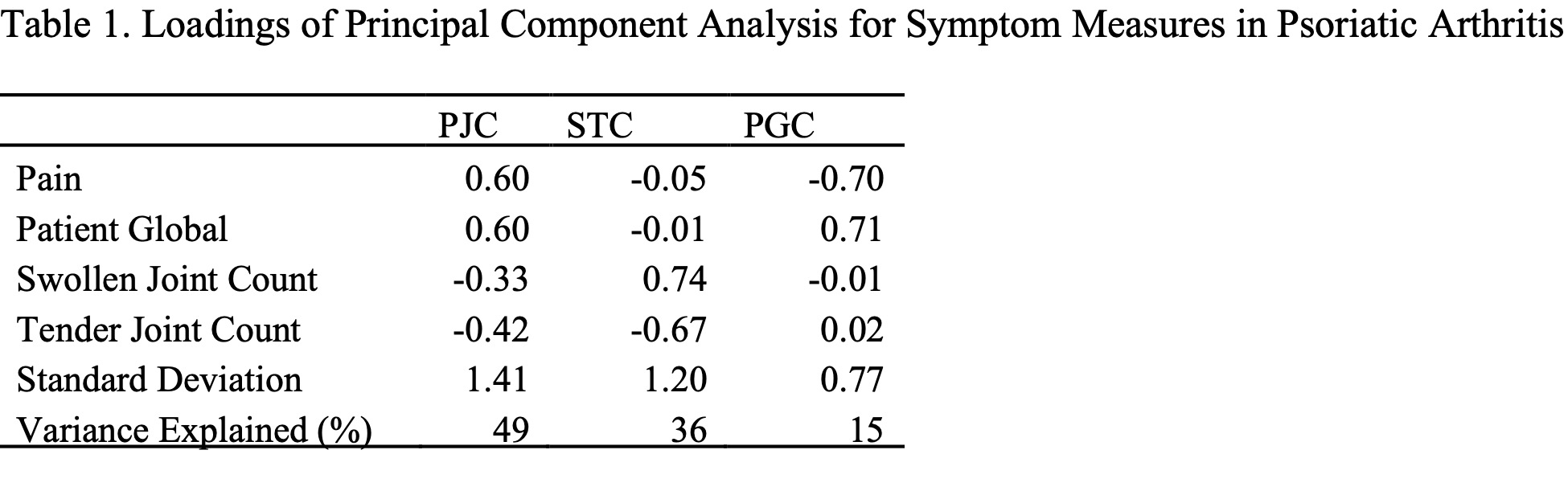 * PRO: patient reported outcome; PJC: PROs and joints contrast; STC: swollen and tender contrast; PGC: pain and global contrast
* PRO: patient reported outcome; PJC: PROs and joints contrast; STC: swollen and tender contrast; PGC: pain and global contrast
.jpg) * NRS: numeric rating scale ; BMI: body mass index; IQI: interquartile interval
* NRS: numeric rating scale ; BMI: body mass index; IQI: interquartile interval
** Percentage may not add up to 100% due to rounding.
*** The Kruskal–Wallis test and Fisher’s exact test were used to compare differences across quartiles for continuous and categorical variables, respectively, due to non-normality of the data.
To cite this abstract in AMA style:
Meng N, Zeger S, Miller J, Haque U, Grader-Beck T, Hummers L, Bingham C, Shah A, Orbai A, Kim J. Enhanced Assessment of Psoriatic Arthritis Disease Progression Using cDAPSA plus the Contrast of Patient Reported Pain and Global Assessment versus Joint Counts [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/enhanced-assessment-of-psoriatic-arthritis-disease-progression-using-cdapsa-plus-the-contrast-of-patient-reported-pain-and-global-assessment-versus-joint-counts/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/enhanced-assessment-of-psoriatic-arthritis-disease-progression-using-cdapsa-plus-the-contrast-of-patient-reported-pain-and-global-assessment-versus-joint-counts/

.jpg)