Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: This study aimed to assess the efficacy of Eltrombopag, a medication used to treat thrombocytopenia, in patients with systemic lupus erythematosus (SLE)-associated thrombocytopenia. Additionally, we investigated the occurrence of thrombotic complications in these patients.
Methods: Nineteen SLE patients with thrombocytopenia were followed for two years or until Eltrombopag discontinuation. Data included medical history, examinations, labs, antiphospholipid serology, response rates, and complications
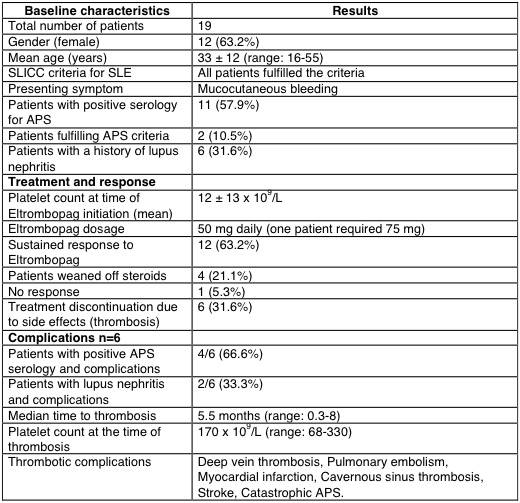
Results: All patients (12 females, mean age 33±12 years) fulfilled the SLICC criteria for SLE and presented with mucocutaneous bleeding. Antiphospholipid serology was positive in 11 patients, with two patients meeting the criteria for antiphospholipid syndrome due to a history of deep vein thrombosis. Six patients had a history of lupus nephritis. Eltrombopag was initiated after the failure of prior therapies. At the start of treatment, the mean platelet count was 12±13 x 109. A daily dose of 50mg of Eltrombopag was used, with one patient requiring 75mg. Twelve patients demonstrated a sustained response, and four patients were able to taper off corticosteroids. However, one patient did not respond to Eltrombopag. Six patients developed thrombotic complications, including deep vein thrombosis, pulmonary embolism, myocardial infarction, cavernous sinus thrombosis, stroke, and catastrophic antiphospholipid syndrome. The median time to thrombotic events was 5.5 months (range: 0.3-8) with a median platelet count of 170 x 109 (range: 68-330). These complications necessitated the discontinuation of Eltrombopag. Four out of the six patients with thrombotic events had positive antiphospholipid antibodies, and two had a history of lupus nephritis.
Conclusion: Our study demonstrates that Eltrombopag is effective in treating SLE-associated thrombocytopenia, with favorable treatment responses observed in the majority of patients, including steroid tapering. However, caution should be exercised regarding the development of thrombotic complications, especially in patients with positive antiphospholipid antibodies. Further research is warranted to identify risk factors and strategies for preventing these complications in this patient population.
To cite this abstract in AMA style:
Beltagy A, Abdelati A, Fayed F, Eshak N, Elghandour A, Halaby M. Eltrombopag in SLE-Associated Thrombocytopenia: Treatment Response and Thrombotic Complications [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/eltrombopag-in-sle-associated-thrombocytopenia-treatment-response-and-thrombotic-complications/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/eltrombopag-in-sle-associated-thrombocytopenia-treatment-response-and-thrombotic-complications/