Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Young adults with childhood-onset lupus (cSLE) are 20 times more likely to die compared to healthy age- and gender-matched counterparts. Their clinical course is more severe than adult-onset lupus, with a 2-3 times increased mortality rate. Poor adherence (i.e. the extent to which an individual’s behavior matches treatment regimens) to therapeutic regimens is a major contributor to negative health outcomes – increased morbidity, mortality, healthcare utilization, and costs. Reliably measuring adherence is critical in targeting this significant problem. However, non-invasive estimates of adherence vary based on measurement method (e.g., self-report, refill data). There is, therefore, a need for objective and reliable methods of measuring adherence to all daily medications. The objective of this study is to examine the feasibility, acceptability, and efficacy of using an electronic pillbox to measure adherence in young adults with cSLE.
Methods: Eleven young adults with cSLE (mean age 20.5±1.6; 36% Caucasian; 55% Black; 1% mixed race) were followed prospectively over 12 weeks. Subjects were recruited during routine pediatric rheumatology clinic visits and met ACR criteria for SLE. All subjects received SimpleMed+ (Viaca, Tel Aviv, Israel) pillboxes that track adherence, for multiple medications and up to four daily doses, using cellular technology. Self-report of adherence using the Medication Adherence Self-Report Inventory (MASRI) was implemented. Additional questionnaires and chart review data were collected. Descriptive analysis and a one-way repeated measures ANOVA were performed.
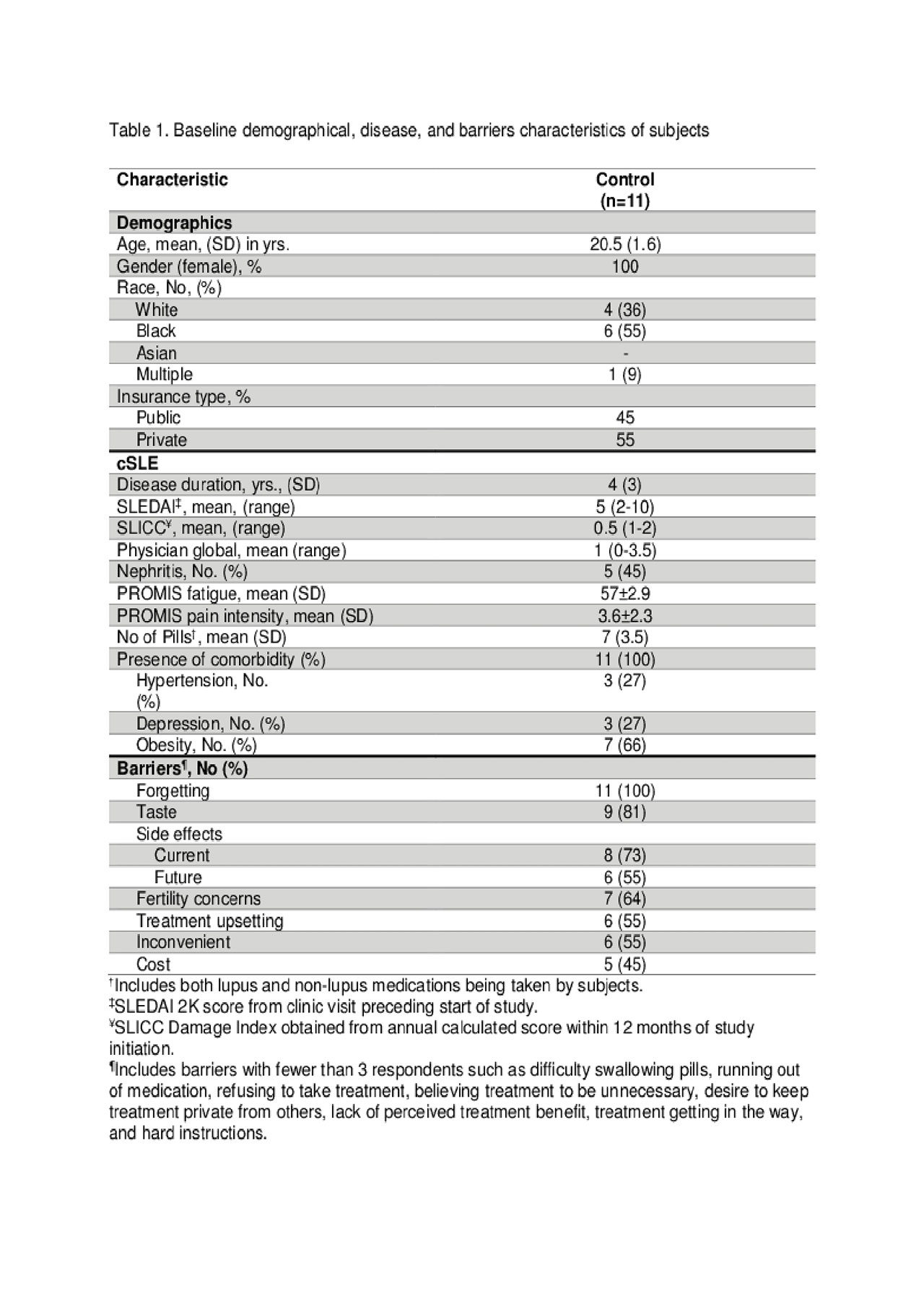
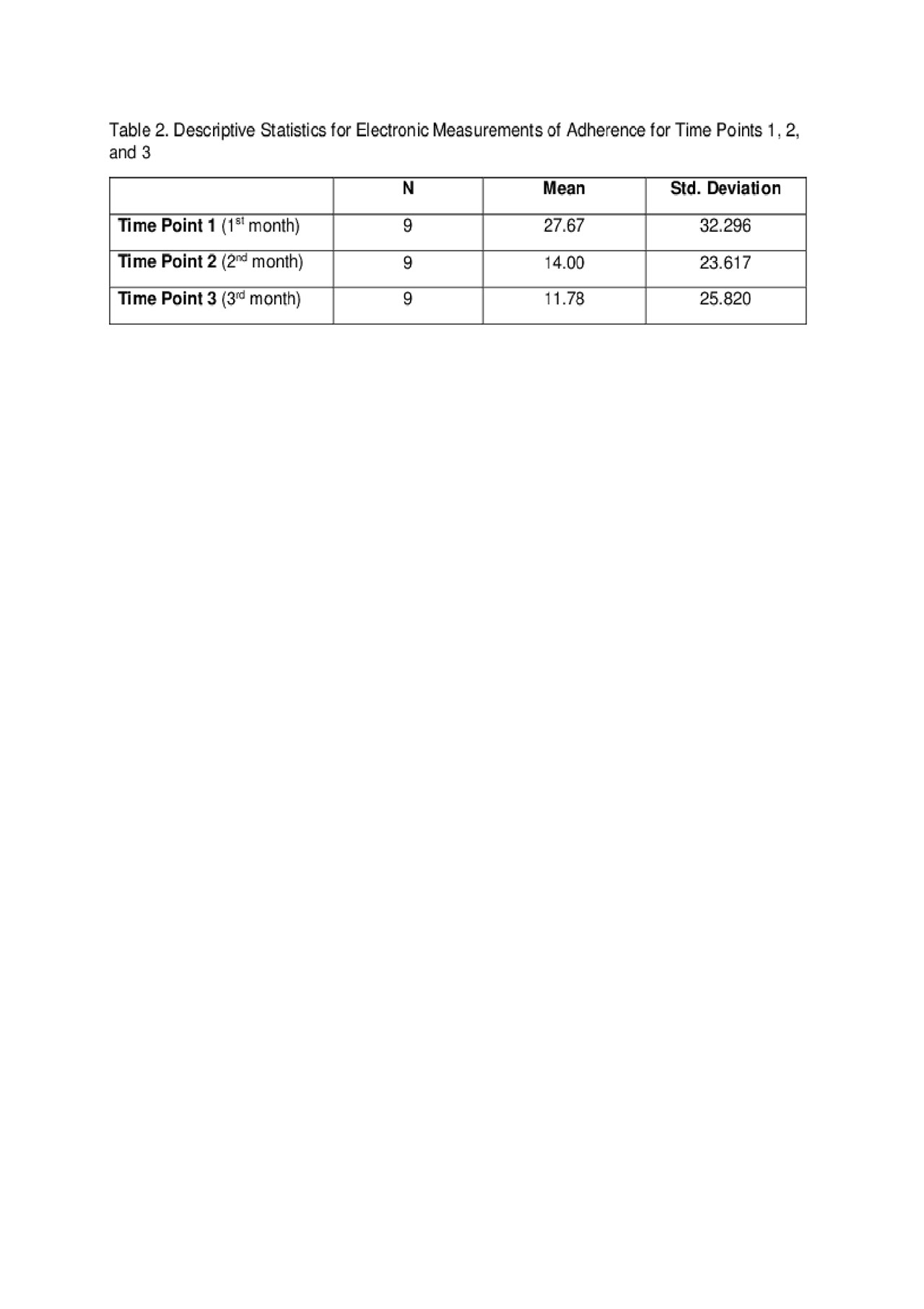
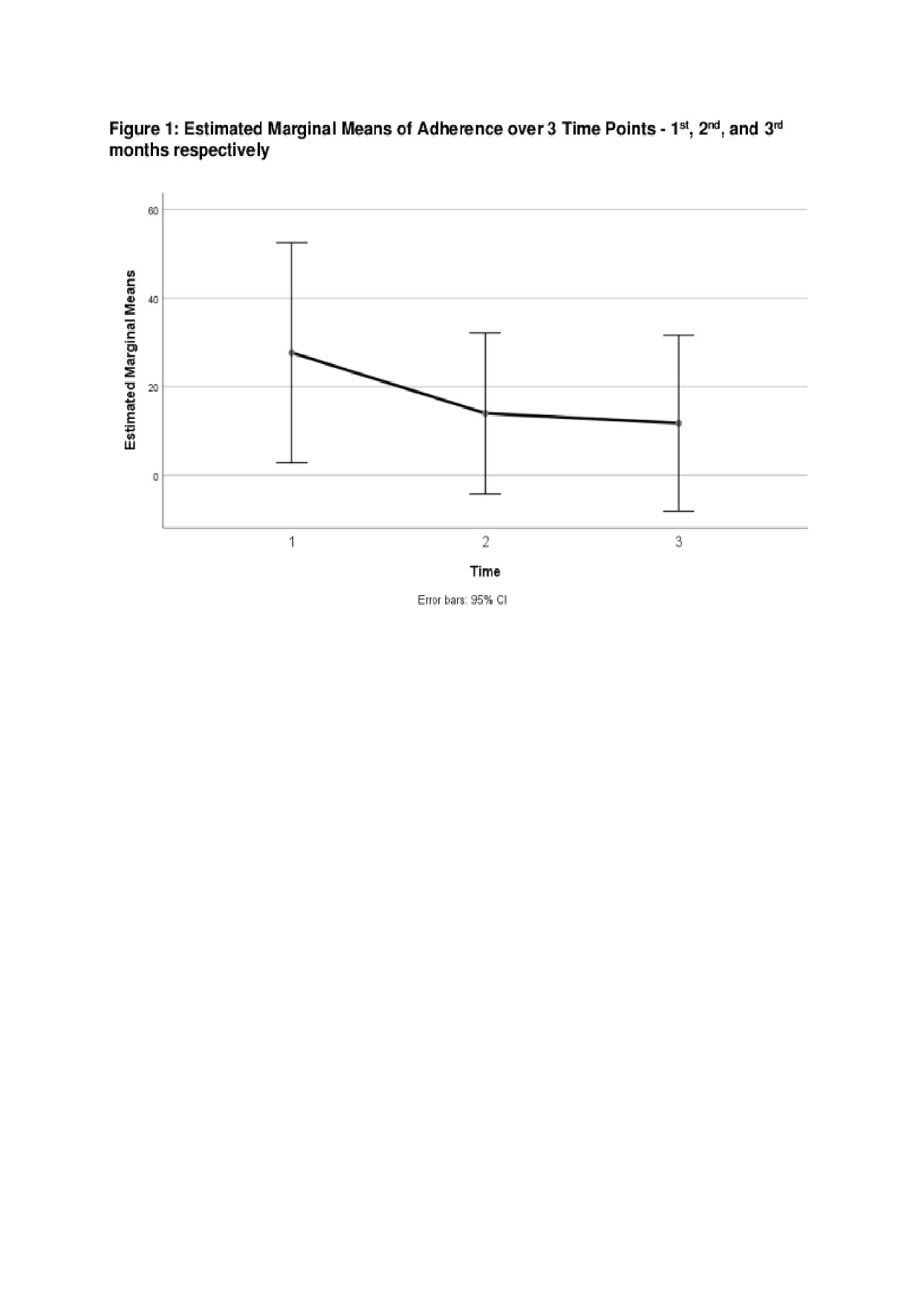
Results: Demographic and disease characteristics are summarized in Table 1. Forgetting, after taste, current medication side effects, and fertility concerns were top barriers to medication adherence (see Table 1). Eighty-one percent of participants (9/11) completed the study and found the pillbox acceptable and easy to use. Reasons for not completing the study were forgetting to refill and/or charging the pillbox. The means and standard deviations of a one-way repeated measure ANOVA (conducted for mean electronic measurements of adherence at 3 time points – 1st, 2nd, and 3rd months) are presented in Table 2. There was no significant effect for time, Wilks’ Lambda = .52, F (2, 7) = 3.24, p = 0.10, multivariate partial դ2 = .48. Although mean self-reported adherence increased from 70.5% to 80% from baseline to study completion, electronically measured adherence decreased over time (Figure 1).
Conclusion: Measuring adherence to multiple medications is feasible with electronic pillboxes. Results from this study suggest that poor medication adherence will not improve without intervention. Consistent with prior studies, our study indicates that self-report of adherence may overestimate true medication adherence. Despite the small sample size, our study show that electronic pillboxes may be a reliable non-invasive method of measuring adherence to all medications taken by patients with cSLE. Establishing a reliable method of measuring adherence to multiple medications is an essential tool for the advancement of adherence research and the ultimate resolution of this significant public health issue.
To cite this abstract in AMA style:
Harry O, Ting T, Huggins J. Electronic Monitoring of Medication Adherence in Young Adults with Childhood-Onset Systemic Lupus Erythematosus over 12 Weeks [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/electronic-monitoring-of-medication-adherence-in-young-adults-with-childhood-onset-systemic-lupus-erythematosus-over-12-weeks/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/electronic-monitoring-of-medication-adherence-in-young-adults-with-childhood-onset-systemic-lupus-erythematosus-over-12-weeks/