Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
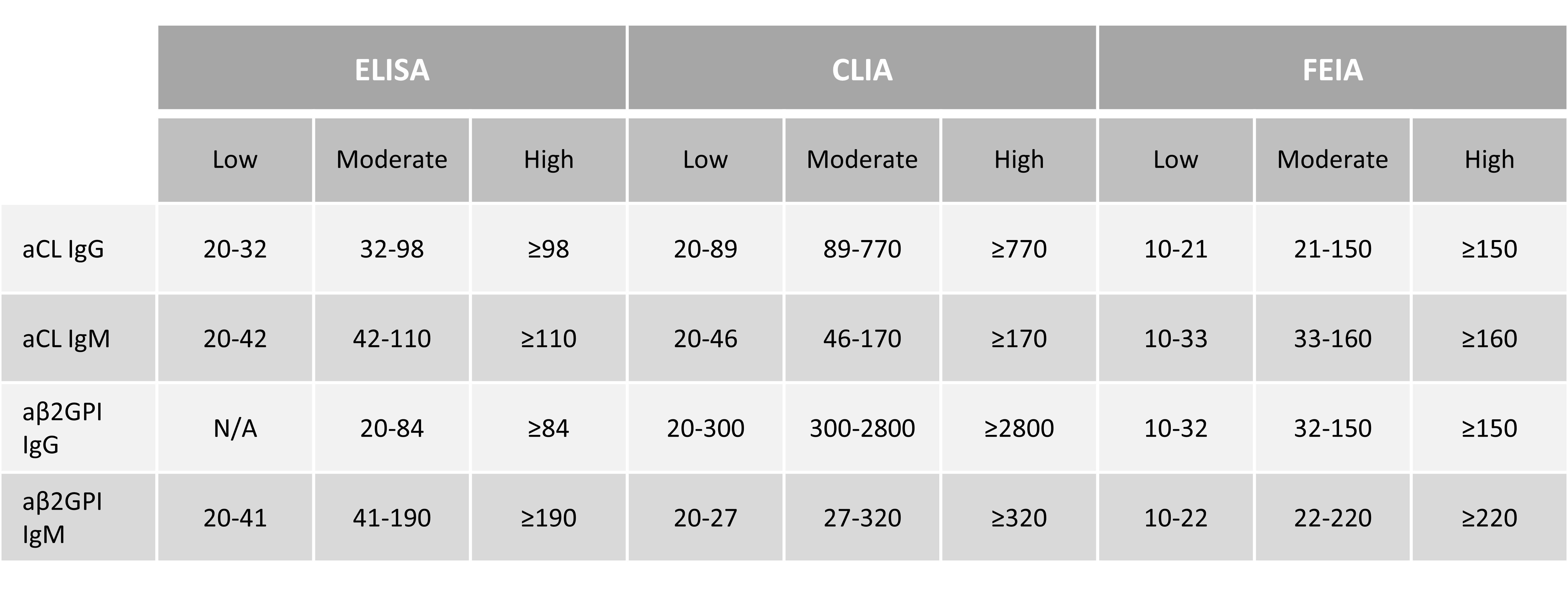
Background/Purpose: Correlation of numerical values between the low, moderate, and high (L, M,H) levels of enzyme-linked immunosorbent assay (ELISA) and non-ELISA platforms for anticardiolipin antibody (aCL) and anti-β2-glycoprotein-I antibody (aβ2GPI) IgG/IgM varies substantially. For instance, based on the International Society on Thrombosis and Haemostasis Scientific and Standardization Committee on Lupus anticoagulant/Antiphospholipid antibodies (ISTH-SSC LA/aPL) Subcommittee multicenter study, an IgG aCL value measured by chemiluminescent immunoassay (CLIA) was up to 5 times higher compared to the ELISA value of 40–80 U (1). Based on this study, L, M, H thresholds for different ELISA and non-ELISA aCL/aβ2GPI tests were previously proposed (2). We aim to determine whether these thresholds can be applied to another independent cohort (AntiPhospholipid Syndrome Alliance For Clinical Trials and InternatiOnal Networking [APS ACTION] registry).
Methods: Patients fulfilling the Revised Sapporo APS Classification Criteria were identified from two independent cohorts: ISTH SSC LA/aPL Multicenter Cohort (Group A) and APS ACTION Registry (Group B). Samples were analyzed in the ISTH-SSC LA/aPL core laboratory (group A) and in APS ACTION validated core laboratories (group B), using QUANTA Lite ELISA (Werfen), AcuStar CLIA (Werfen) and Phadia EliA fluorescence enzyme immunoassay (FEIA) (Thermo Fisher). Thresholds for L-M-H positive aPL levels previously determined by receiver operating characteristic (ROC) analysis correspond to three ranges (Manufacturer’s cutoff – 97.5% specificity, 97.5% – 99.5% specificity, and ≥99.5% specificity) (Table 1) [1]. The frequency of aCL/aβ2GPI positivity and the distribution in L-M-H levels were compared between the two cohorts. Statistical analyses (box-and-whisker plots, Mann-Whitney U-testing, comparison of proportions and Chi-Square statistics) were done with Python and MedCalc.
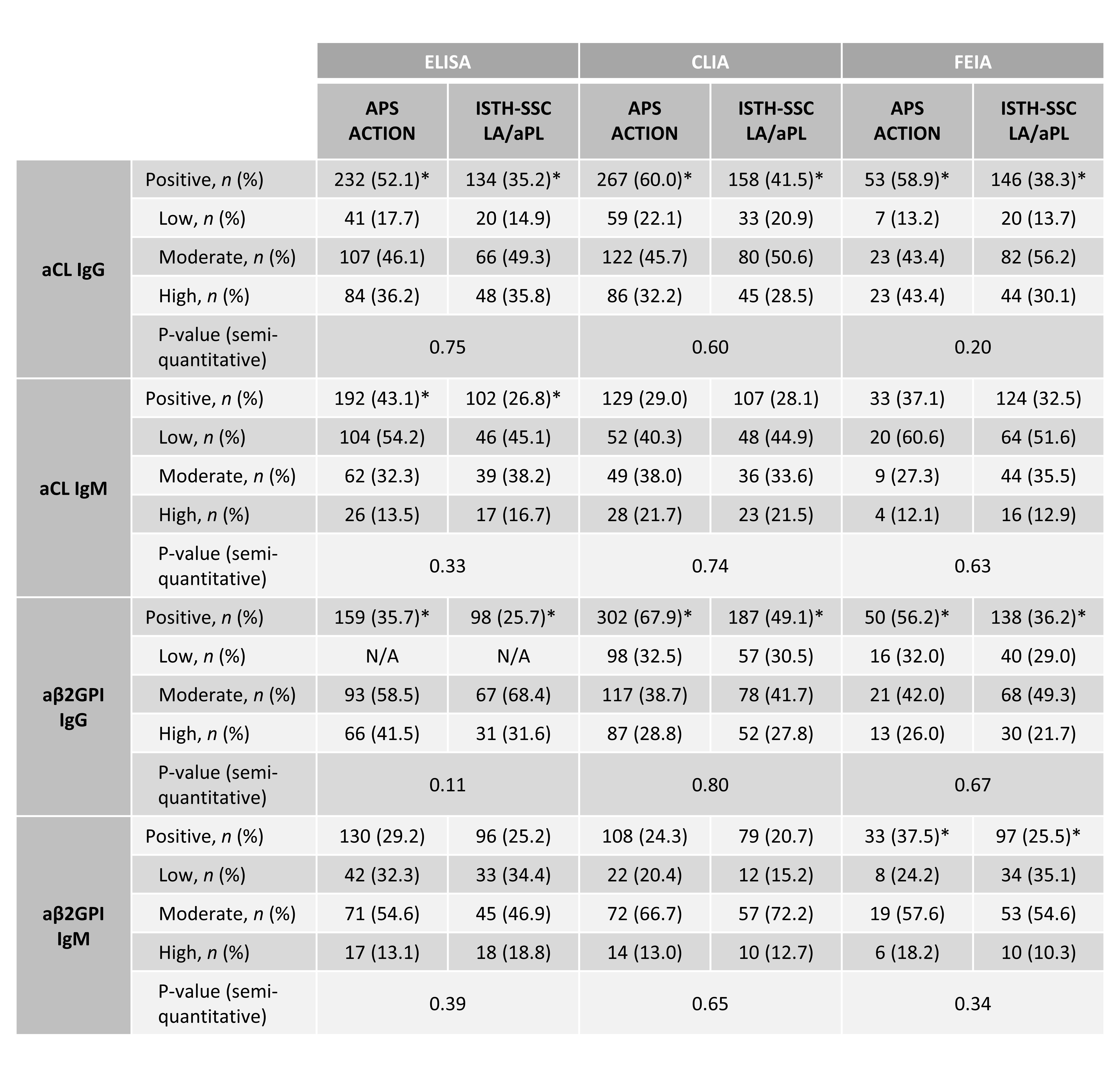
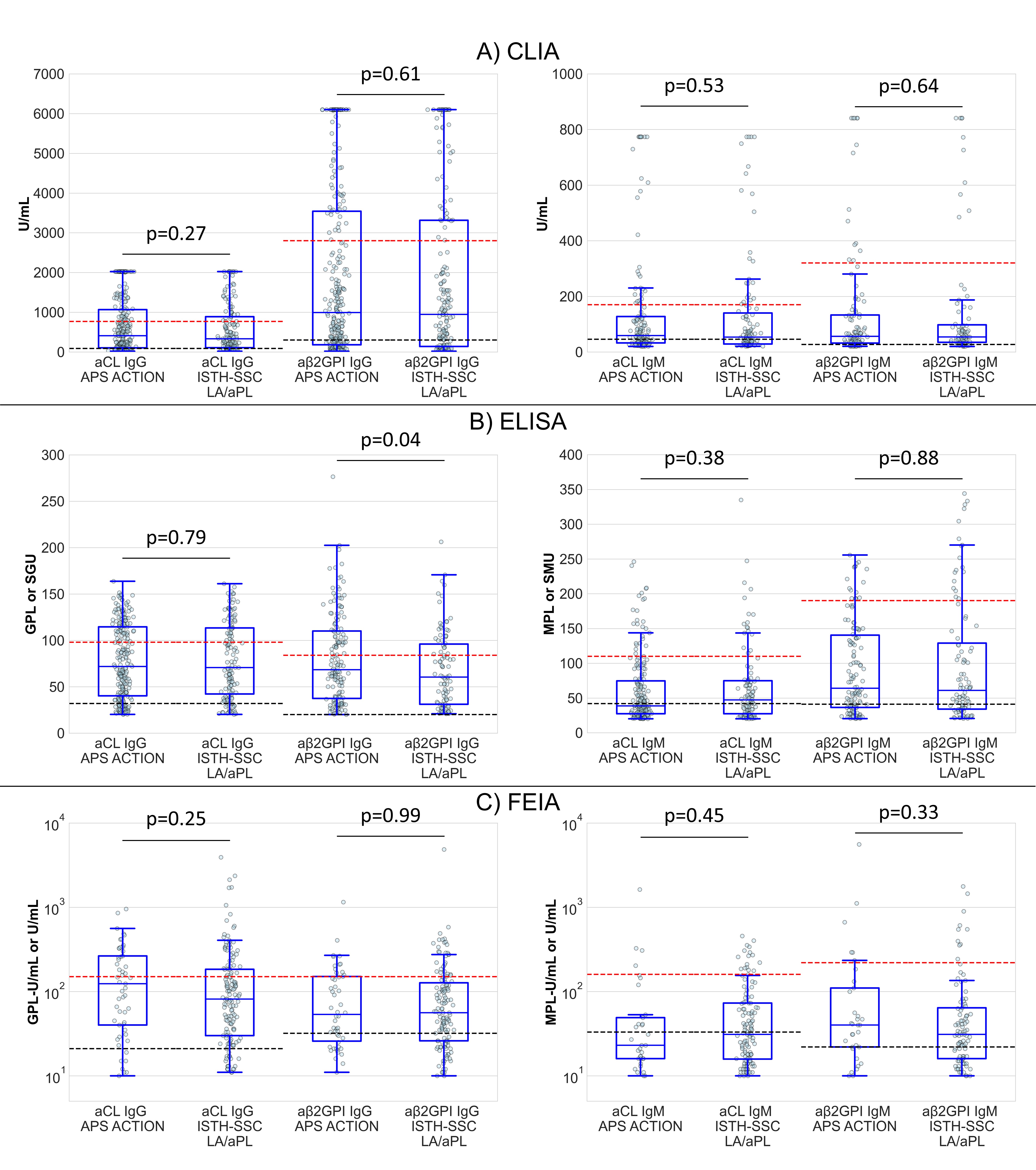
Results: Based on the analysis of 381 Group A (ISTH) and 445 Group B (APS ACTION) patients, group B patients were more frequently positive for aCL/aβ2GPI, compared to Group A (Table 2) but with similar aPL levels except for aβ2GPI IgG (Figure 1). aCL and aβ2GPI IgG/IgM results had similar proportions within the L-M-H semiquantitative ranges in the two patient cohorts (Table 2).
Conclusion: In APS patients, the distribution of positive aCL and aβ2GPI IgG/IgM categorized into L-M-H levels, measured by ELISA, CLIA and FEIA, was similar between two independent patient cohorts. These results suggest that thresholds for semiquantitative interpretation of aCL and aβ2GPI results could apply across laboratories and APS patient populations.
[1] Vandevelde, A., et al. J Thromb Haemost, 2022, doi: 10.1111/jth.15585
[2] Vandevelde, A., et al. J Thromb Haemost, 2024, doi: 10.1016/j.jtha.2024.04.016
P-values derived from Chi-Square tests; * denotes significant different proportion positives (p<0.05); N/A = Not applicable.
P-values derived from Mann-Whitney U tests; black and red dotted lines represent respective moderate and high level thresholds. For FEIA, the y-axis has a logarithmic scale.
To cite this abstract in AMA style:
Vandevelde A, Meroni P, Cohen H, Andrade D, Amengual O, ATSUMI T, Tincani A, Belmont H, Borghi M, Branch D, Cervera R, Ramires de Jesús G, Fortin P, Gris J, Grossi C, Knight J, Moore G, Musiał J, Petri M, Rodriguez-Almaraz E, Paredes-Ruiz D, Roubey R, Tebo A, Tektonidou M, WAHL D, Zuily S, Willis R, Pengo V, Bertolaccini M, Erkan D, Devreese K. Efforts to Harmonize ELISA and Non-ELISA Anticardiolipin and Anti-β2-glycoprotein-I Levels Based on ISTH SSC LA/aPL and APS ACTION International Multicenter Cohorts [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efforts-to-harmonize-elisa-and-non-elisa-anticardiolipin-and-anti-%ce%b22-glycoprotein-i-levels-based-on-isth-ssc-la-apl-and-aps-action-international-multicenter-cohorts/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efforts-to-harmonize-elisa-and-non-elisa-anticardiolipin-and-anti-%ce%b22-glycoprotein-i-levels-based-on-isth-ssc-la-apl-and-aps-action-international-multicenter-cohorts/