Session Information
Date: Tuesday, October 23, 2018
Title: Systemic Lupus Erythematosus – Clinical Poster III: Treatment
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: To investigate effectiveness, safety, and predictors of response to belimumab in patients with active SLE in clinical practice setting.
Methods: Four hundred and one active SLE patients (ACR criteria) with positive anti-dsDNA antibody and low C3 and/or C4, from 22 Italian Centers, were treated with belimumab (10 mg/kg day 0, 14, 28 and then every 28 days), as add-on therapy. They were 366 (91.27%) females, mean age 43.51±11.20 years; mean disease duration 12.79±8.74 years.
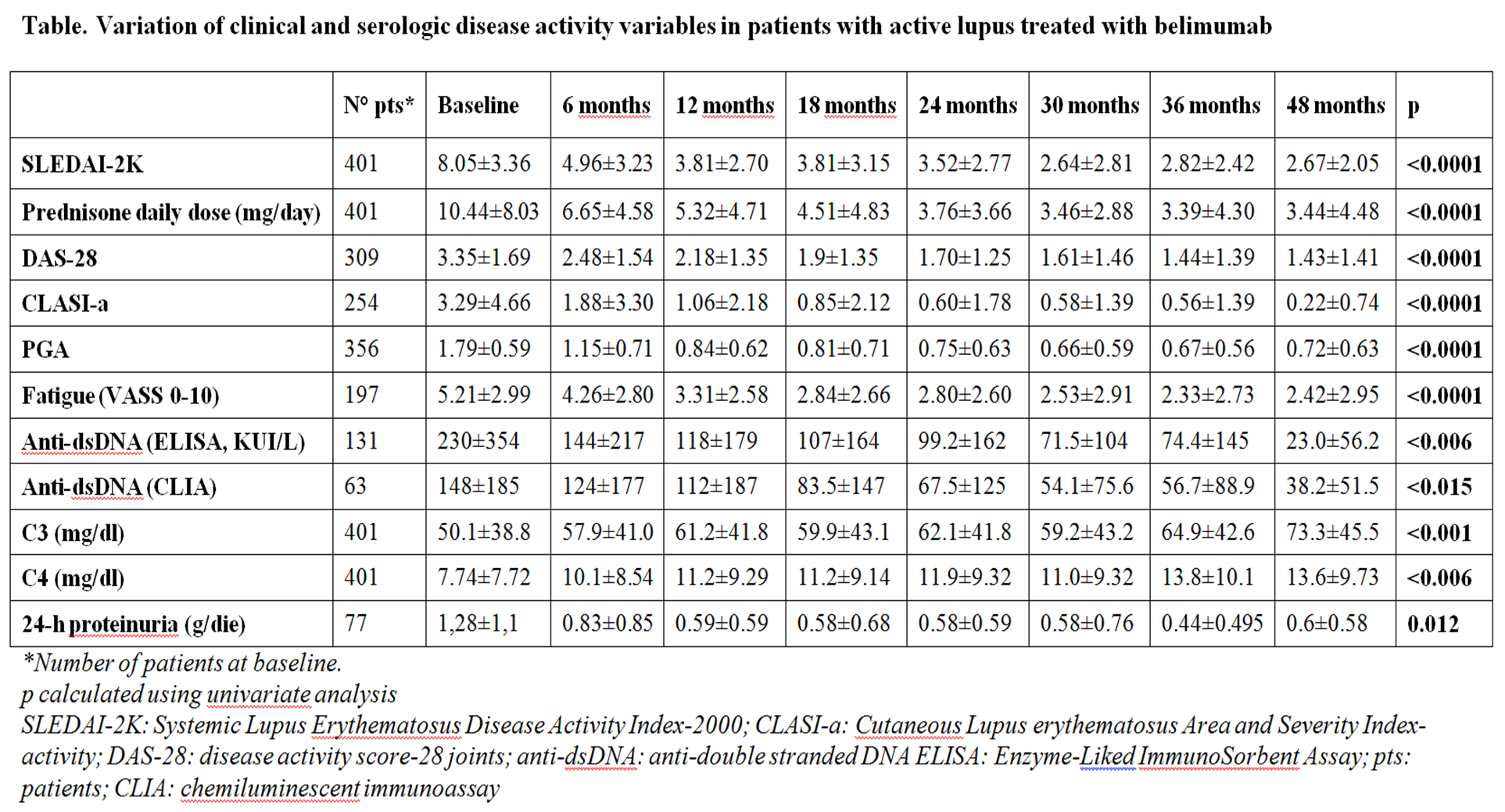
SLEDAI-2K, anti-dsDNA, C3, C4, prednisone daily dose, DAS-28, 24-hours proteinuria, CLASIa (Cutaneous LE Disease Area and Severity Index Activity), PGA, Fatigue (VAS 0-10) were recorded at baseline and every 6 months. SLICC-DI was evaluated 5-years before belimumab initiation, at baseline and every year after belimumab initiation. Response was evaluated according to SLE Responder Index-4 (SRI-4) at 12, 24, 36 and 48 months. Adverse events (AEs) were subdivided in noninfectious or infectious AE, and infusion reactions. AE was defined as severe (SAE) when hospitalization was required and/or death and/or life-threatening manifestations occurred. Statistics were performed by pairs T-test, chi-square test and multiple logistic regression analysis using SPSS package (version 22.0).
Results: Mean follow-up was 21.07±15.34 months (range 3-60). Active manifestations requiring the use of belimumab were articular in 40.82%, mucocutaneous in 21.84%, renal in 14.56%, hematologic in 16.32% and serositis in 6.24% of cases. SRI-4 was achieved by 68.2%, 73.3%, 74.7% and 68.3% of patients at 12, 24, 36 and 48 months, respectively. The improvement in clinical and serological variables during the follow-up are reported in the Table.
We observed a decrease in the mean number of flare during belimumab treatment compared with the corresponding period before belimumab initiation (p<0.0001).
SLEDAI-2K ≥10 resulted as an independent predictor of response by logistic regression at month 12 and 24 (p=0.003 and p=0.025).
Mean SLICC-DI was 0.71±1.07 five years before belimumab initiation, 0.96±1.31 at baseline and 1.00±1.52, 1.09±1,70, 1.27±1.91 at 24, 36 and 48 months of follow-up, respectively. We observed a significant increase in mean SLICC-DI between 5 years before belimumab initiation and baseline (p<0.01), but not between baseline and 2-year follow-up.
A total of 8,831 infusions were analyzed. A total of 681 AEs were observed in 298 patients, SAEs were 32 in 29 patients. No severe infusion reactions were observed; 15 patients had infective SAEs, and 18 non infective SAEs.
Conclusion: Belimumab reduces global and organ specific disease activity, frequency of flares and prednisone daily dosage. SLDAI-2K ≥10 at baseline was the best predictor of response in our cohort of patients with active SLE. Belimumab was well tolerated.
To cite this abstract in AMA style:
Iaccarino L, Saccon F, Mathieu A, Piga M, Ceribelli A, Selmi C, Cardinaletti P, Gabrielli A, Di Matteo A, De Angelis R, Faggioli P, Laria A, Fredi M, Regola F, Tincani A, Andreoli L, Pazzola G, Salvarani C, Puppo F, Magnani O, Prete M, Gremese E, Gerosa M, Ubiali T, Bozzolo E, Canti V, Racanelli V, Conti F, Ceccarelli F, Bartoloni E, Gerli R, Lobasso A, de Paulis A, De Marchi G, De Vita S, Bortoluzzi A, Govoni M, Benvenuti F, Zen M, Mosca M, Tani C, Rossini M, Orsolini G, Scarpato S, Brunetta E, Zumbo A, Tanti G, Doria A. Efficacy, Safety, and Predictor of Response to Belimumab in a Large Nationwide Cohort Study of Patients with Active Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/efficacy-safety-and-predictor-of-response-to-belimumab-in-a-large-nationwide-cohort-study-of-patients-with-active-systemic-lupus-erythematosus/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-safety-and-predictor-of-response-to-belimumab-in-a-large-nationwide-cohort-study-of-patients-with-active-systemic-lupus-erythematosus/