Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Etanercept (ETN) is effective and well tolerated in patients with non-radiographic axial spondyloarthritis (nr-axSpA). However, there is limited information on the effects of withdrawing ETN treatment in patients who have achieved a significant clinical response. RE-EMBARK was a phase 4, multicenter, open-label, 3-period study that evaluated the effects of ETN withdrawal and retreatment on clinical efficacy in patients with nr-axSpA. The aim of this analysis was to evaluate differences in outcomes during treatment withdrawal (Period 2) based on sustained remission status during the active treatment phase (Period 1).
Methods: RE-EMBARK enrolled patients aged 18–49 years with active nr-axSpA who had an inadequate response to ≥2 nonsteroidal anti-inflammatory drugs (NSAIDs) and were taking a stable dose of an NSAID for at least 14 days before their first study dose of ETN. In Period 1, all patients received open-label ETN 50 mg once weekly and stable background NSAID for 24 weeks. Patients who achieved inactive disease (ASDAS-CRP < 1.3) at Week 24 were eligible to enter Period 2. In Period 2, ETN was withdrawn for up to 40 weeks. Patients with an nr-axSpA flare (ASDAS-ESR ≥2.1) during Period 2 were eligible to immediately enter Period 3. In Period 3, patients were retreated with open-label ETN 50 mg once weekly for 12 weeks. In this post-hoc analysis, sustained response (SR) during Period 1 was defined as ASDAS ESR inactive disease at week 24 and at either Week 12 or Week 16, and no flare at any of these time points.
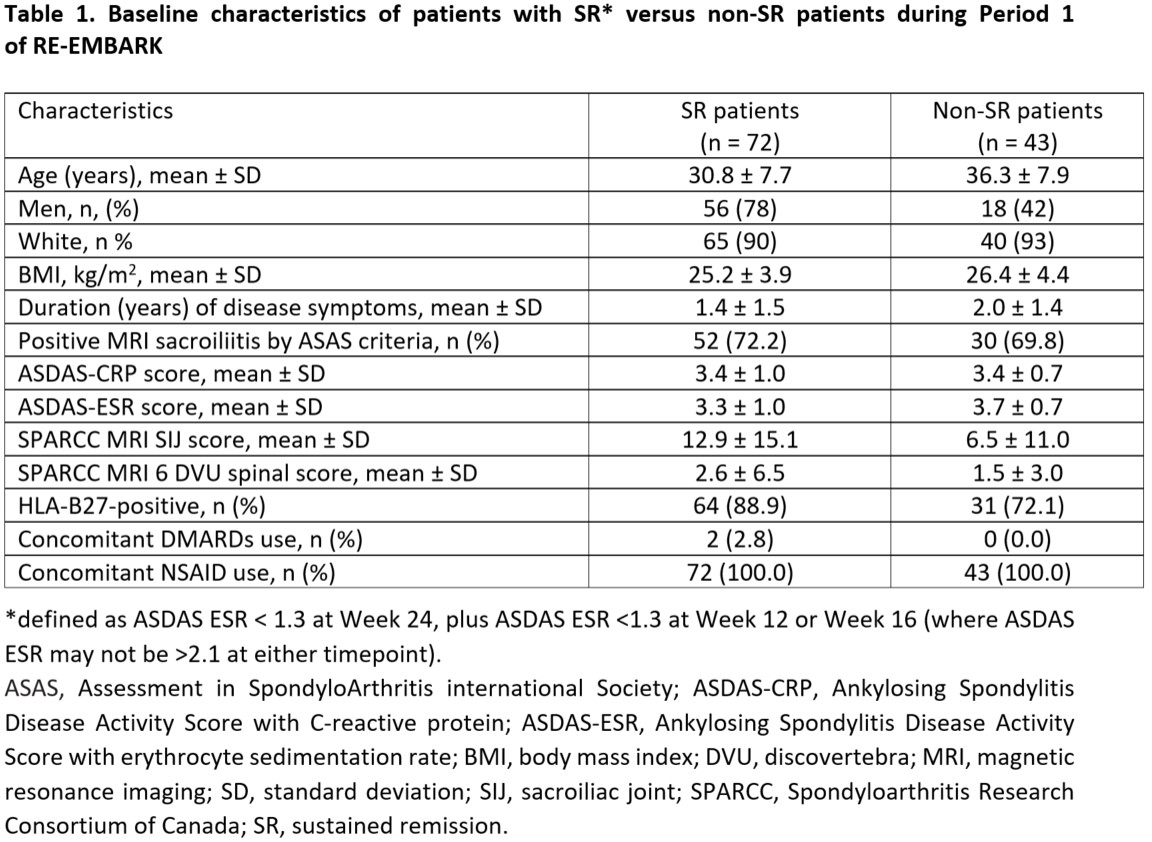
Baseline demographic and clinical characteristics of SR patients were compared with non-SR patients. Statistical analyses comparing SR versus non-SR patients were conducted using logistic models for binary endpoints and ANCOVA for continuous endpoints.
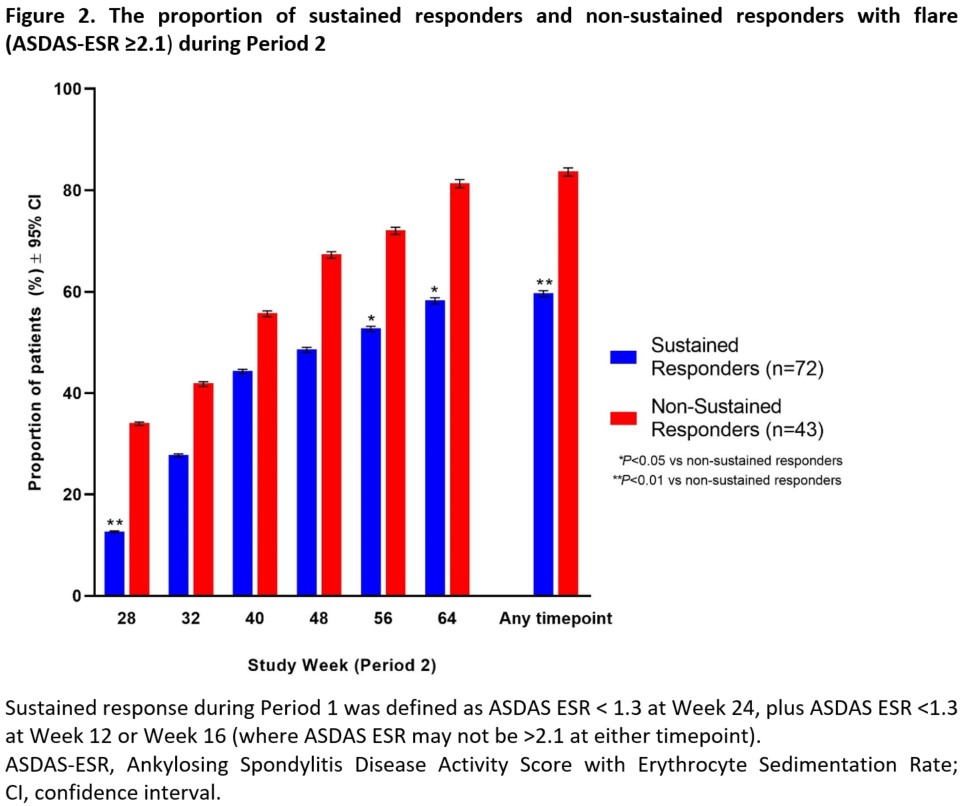
Results: A total of 209 patients were treated during Period 1, of which 119 (57%) achieved inactive disease and entered Period 2 (ETN treatment withdrawal). A total of 115 patients who had at least one efficacy evaluation during Period 2 were included in this post-hoc analysis, with 72 patients achieving SR versus 43 patients with non-SR. Period 1 SR patients were younger, had a higher SpondyloArthritis Research Consortium of Canada MRI sacroiliac joint score SIJ score, and were more likely to be HLA-B27 positive than non-SR patients (Table 1). In Period 2, Period 1 SR patients showed greater reductions in ASDAS-CRP than non-SR patients, which was statistically significant at Weeks 28, 32, 48, 56, and 64 (Figure 1). A significantly smaller proportion of Period 1 SR versus non-SR patients had a flare during Period 2 at Weeks 28, 56, 64, and all timepoints combined (Figure 2). When all timepoints were combined, 59.7% of Period 1 SR patients had a flare compared with 83.7% of non-SR patients.
Conclusion: Patients who demonstrated SR to ETN in Period 1 showed greater prolongation of efficacy and fewer flares when ETN treatment was withdrawn (Period 2) compared with non-SR patients. These results suggest on-treatment efficacy can affect how patients respond to withdrawal of ETN.
To cite this abstract in AMA style:
Van den Bosch F, Nash P, Wei J, Blanco F, Tsekouras V, Zang C, Graham D, Arthur E, Selema P, Vlahos B, Deodhar A. Efficacy Outcomes Following Etanercept Withdrawal by Sustained Remission Status in Patients with Nr-axSpA: Results from RE-EMBARK [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-outcomes-following-etanercept-withdrawal-by-sustained-remission-status-in-patients-with-nr-axspa-results-from-re-embark/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-outcomes-following-etanercept-withdrawal-by-sustained-remission-status-in-patients-with-nr-axspa-results-from-re-embark/