Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Low back pain (LBP) is a common and debilitating problem. There are currently very limited pharmacological treatments for LBP. Vertebral endplate subchondral bone abnormalities (Modic abnormalities (MC)) are consistently associated with LBP and could be a potential treatment target. Previous research suggests that bone active agents, such as zoledronic acid (ZA) may be effective for knee bone marrow lesions and back pain. No evidence is available for denosumab (DN). This study aims to compare the effects of ZA and DN on LBP in patients with MC.
Methods: Adults aged ≥40 years with significant LBP (>6 months) and MC (type 1, 2 or mixed) were randomised to receive either ZA (5mg/100ml) or DN (60mg), or placebo. The chief outcomes were change in pain assessed by Visual Analogue Scale (VAS, 0-100) and size of MC measured on MRIs of T12-S1 vertebrae over 6 months. Other outcomes included: change in pain assessed by the LBP Rating Scale (RS, 0-30), disability by the Roland-Morris Disability Questionnaire (RMDQ, 0-24), and quality of life by Assessment of Quality of Life (AQoL, 0-48) after 3 and 6 months. Repeated measures regression was performed to analyse the change in outcomes.
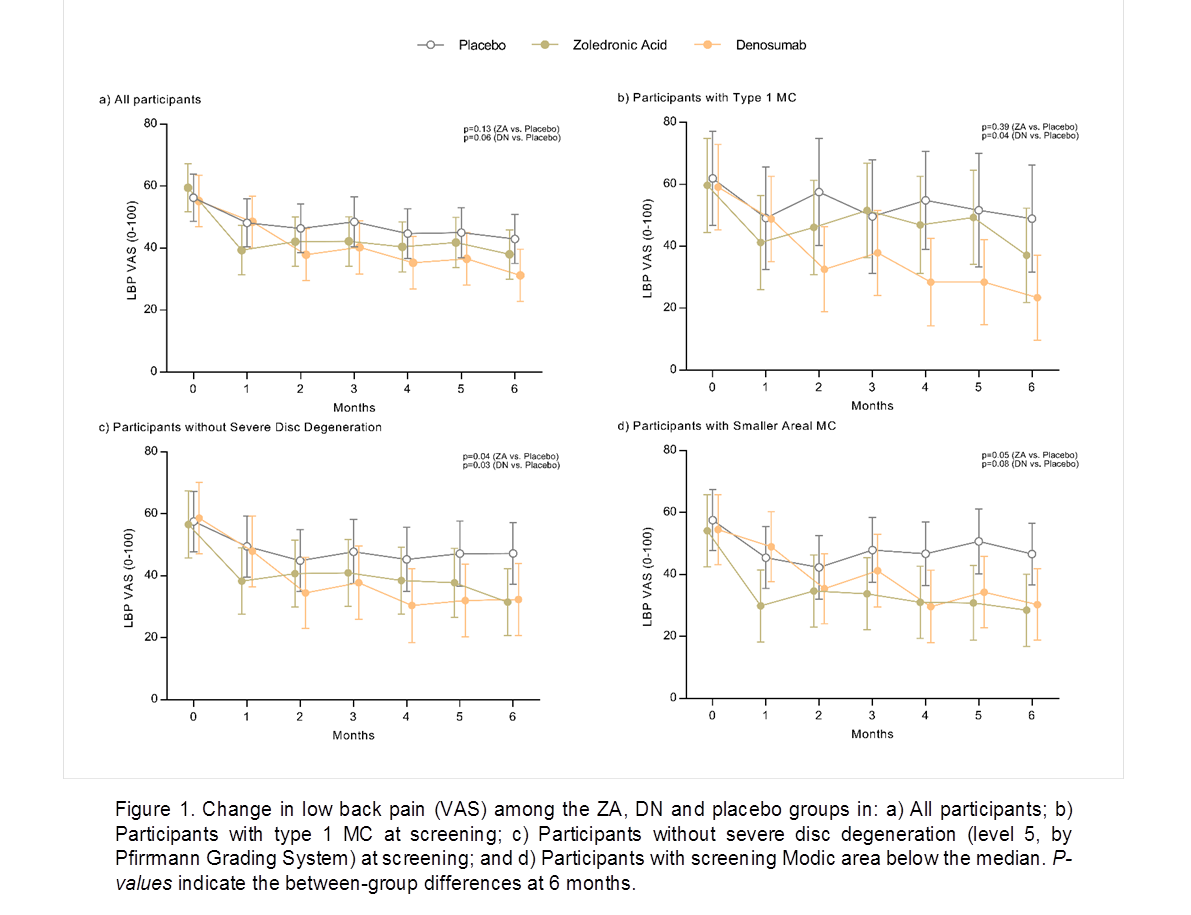
Results: 103 participants (39% females, mean age 59.8 yrs) were enrolled. At baseline, mean (SD) VAS, and RS scores were 57.1 (18.3) and 17.6 (5.0), and the median total MC area was 538 mm2. Subjects were generally well matched at baseline. Table 1 presents the findings for the chief and other outcomes. Compared to placebo, VAS scores decreased by clinically significant amounts in both the ZA and DN groups after 6 months, but the change was not statistically significant (p=0.13 and 0.06 respectively). There was little reduction in areal MC size and no difference between groups. LBP RS scores were significantly reduced compared to placebo in the ZA group after 3 (-3.5, 95%CI -6.2 to -0.8) and 6 months (-3.3, 95%CI -5.9 to -0.7), and in the DN group after 6 months (-3.0, 95%CI -5.7 to -0.3). Improvements in disability (RMDQ) occurred in the ZA group after 3 months (-2.1, 95%CI -4.0 to -0.2). Time course analysis suggested ZA treated patients had lower VAS pain scores after one month and DN after 2 months. Both therapies were more effective for VAS pain after 6 months in those with smaller Modic area and milder disc degeneration as classified at screening, and DN was more effective for VAS pain in patients with type 1 MC (Fig 1). Adverse events were more frequent in the ZA group; primarily flu-like symptoms and headaches.
Conclusion: These pilot findings suggest that both ZA and DN may reduce LBP associated with MC but do not change MC size over 6 months.
To cite this abstract in AMA style:
Cai G, Laslett L, Aitken D, Halliday A, Pan F, Otahal P, Speden D, Winzenberg T, Jones G. Efficacy of Zoledronic Acid and Denosumab in the Treatment of Patients with Low Back Pain and Modic Changes: A Proof of Principle Trial [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-zoledronic-acid-and-denosumab-in-the-treatment-of-patients-with-low-back-pain-and-modic-changes-a-proof-of-principle-trial/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-zoledronic-acid-and-denosumab-in-the-treatment-of-patients-with-low-back-pain-and-modic-changes-a-proof-of-principle-trial/