Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Patients with psoriatic arthritis (PsA) who experience anti-TNF failure and switch to another TNF inhibitor as second- or third-line therapy typically show poorer responses compared to those receiving first-line anti-TNF treatment.1 In this post-hoc analysis, we assessed baseline characteristics and long-term efficacy outcomes with upadacitinib (UPA) in the subgroup of patients with any prior anti-TNF exposure compared to the overall population within the SELECT-PsA 2 study, as well as explored efficacy by number of prior anti-TNF therapies.
Methods: In SELECT-PsA 2 (a double-blind, randomized controlled phase 3 trial), patients with active PsA and inadequate response or intolerance to ≥1 biologic DMARD were treated with UPA 15 mg or 30 mg once daily or placebo (PBO) for 24 wks.2 Patients who had previous exposure to any JAK inhibitor were excluded. Stable background treatment with ≤2 non-biologic DMARDs was permitted but not required. Only data from patients initially randomized to UPA 15 mg are included here. This analysis determined the proportion of patients achieving ACR20/50/70, PASI90, resolution of dactylitis or enthesitis, and Minimal Disease Activity (MDA) up to wk 104 in both the overall study population and in the subgroup of patients with prior anti-TNF exposure (stratified by any exposure to TNF inhibitors and by number of prior anti-TNF treatments). NRI was used for all binary endpoints.
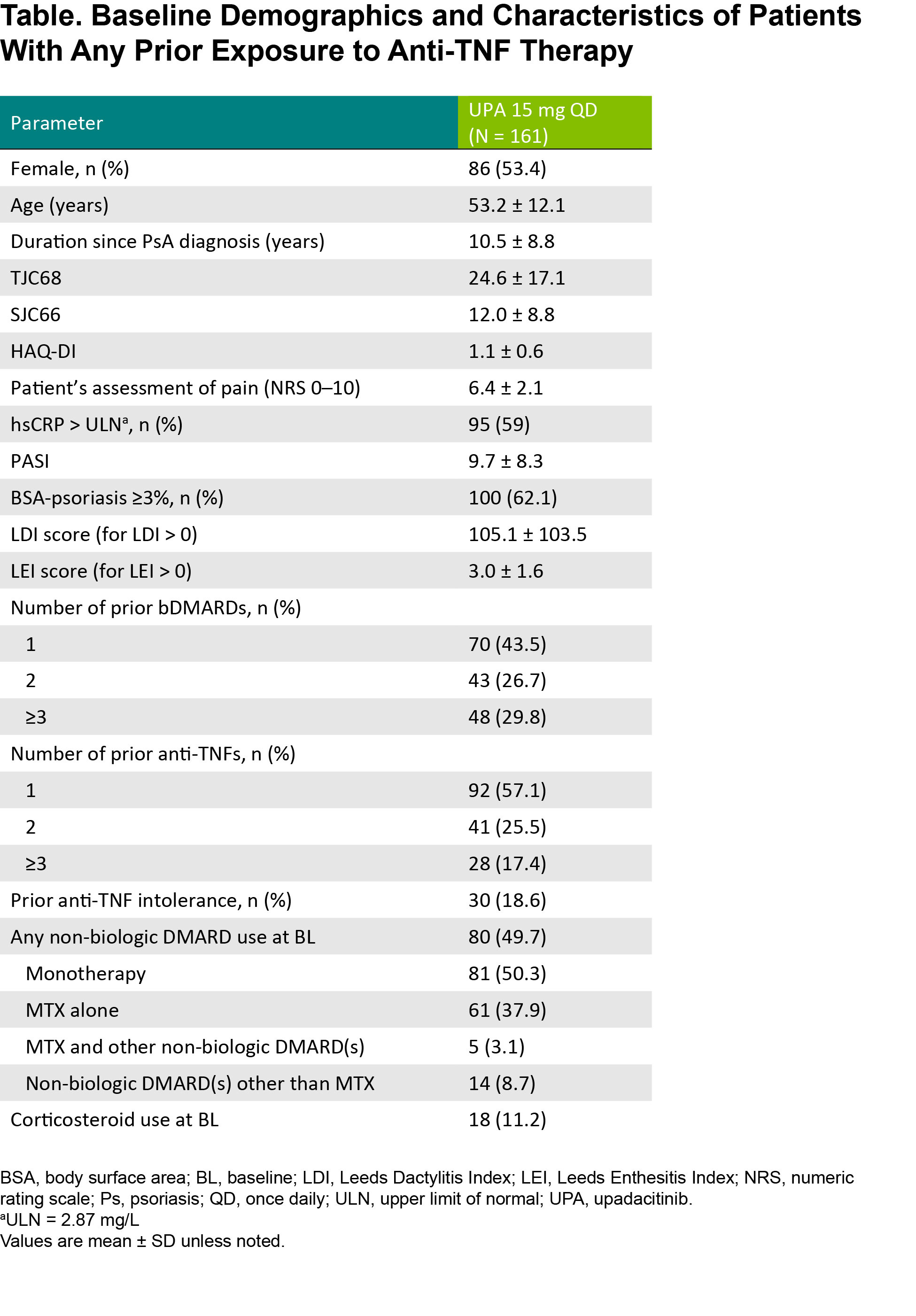
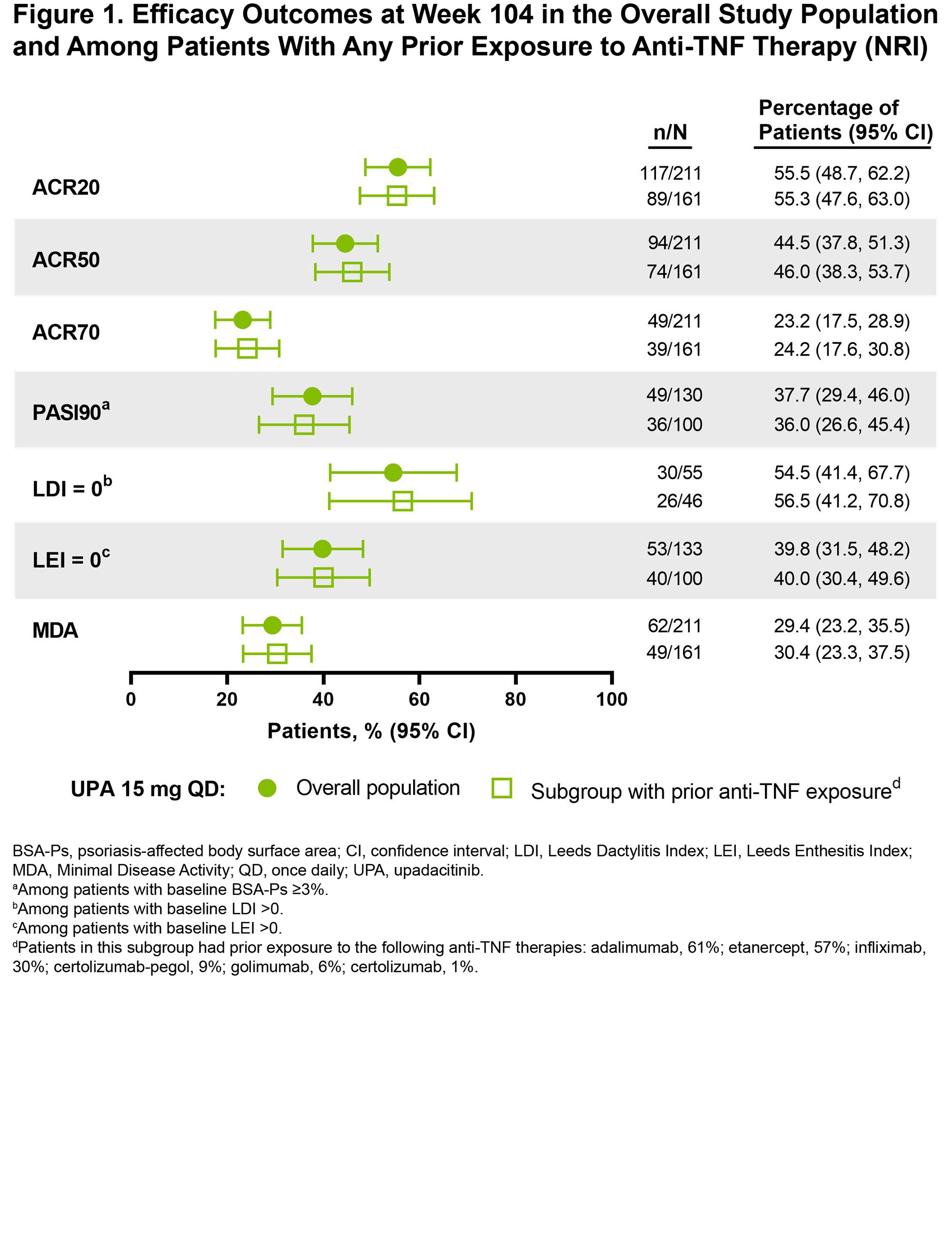
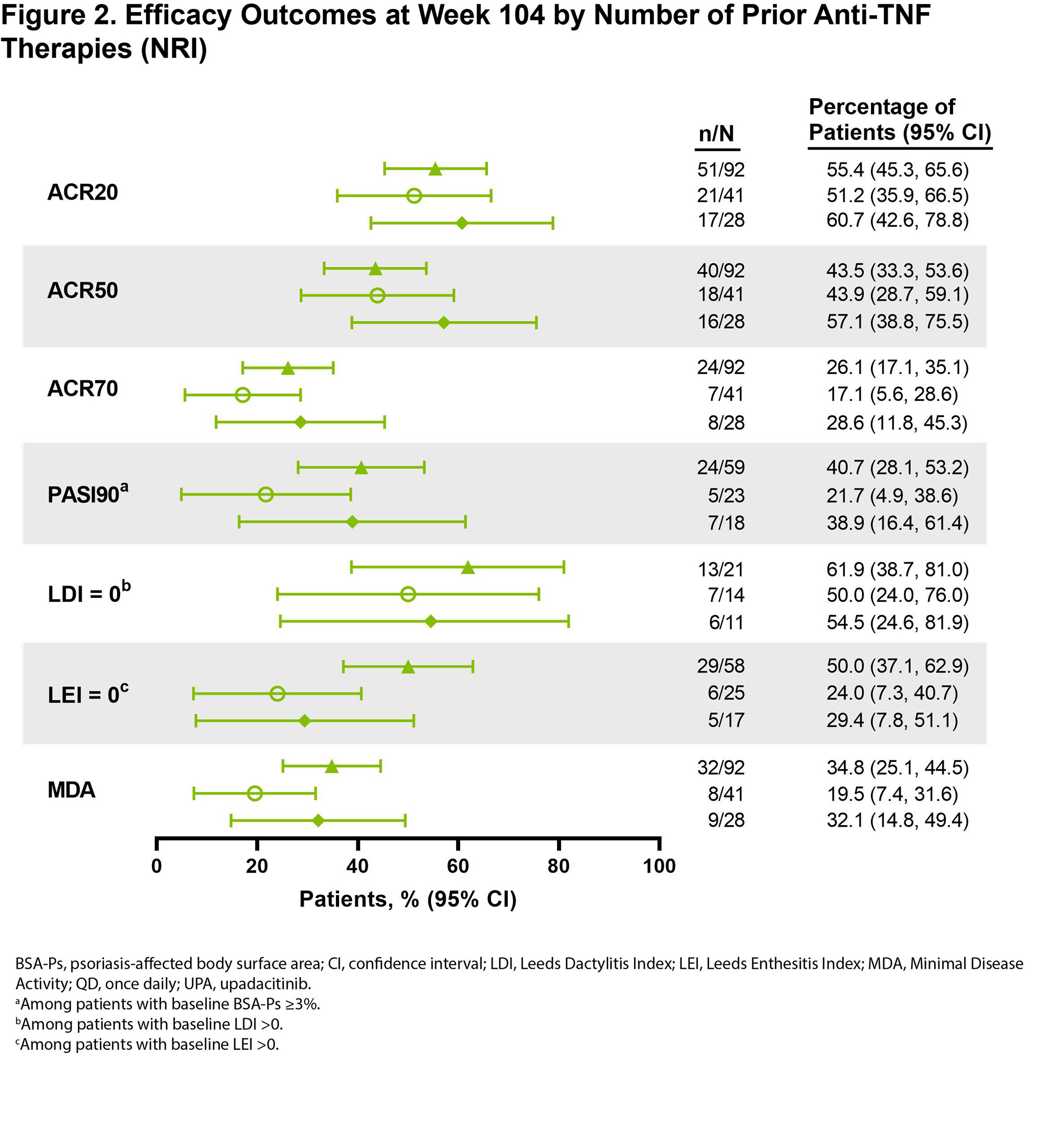
Results: Among patients randomized to UPA 15 mg (n = 211) in SELECT-PsA 2, 161 had prior exposure to any anti-TNF therapy. Baseline demographics and disease characteristics, including age, sex, years since PsA diagnosis, and use of background PsA therapies, were similar between the subgroup with anti-TNF exposure (Table) and the overall study population as reported previously.2 Most patients in the anti-TNF exposure subset received 1 prior TNF inhibitor (57%), while 26% previously received 2 anti-TNFs and 17% previously received ≥3 anti-TNFs; 19% of patients were anti-TNF intolerant. At baseline, 50% and 11% of patients in the subgroup received concomitant non-biologic DMARD(s) and/or corticosteroid therapy, respectively. Efficacy outcomes were generally consistent among patients in the overall study population and in the subset of patients with prior anti-TNF exposure (Figure 1). Across efficacy endpoints, UPA 15 mg treatment also led to similar responses in patients with prior exposure to 1, 2, or ≥3 anti-TNF therapies (Figure 2). Given the limited sample sizes for the subset of patients with ≥3 prior anti-TNFs, however, those results should be interpreted with caution, but it is nonetheless noteworthy that this more refractory population demonstrated such favorable responses.
Conclusion: The prior anti-TNF exposure subgroup receiving UPA 15 mg demonstrated consistent efficacy in treating PsA (including joint-related symptoms, psoriasis, dactylitis, enthesitis, and comprehensive disease control as assessed by MDA) compared to the overall population in SELECT-PsA 2, regardless to the number of prior anti-TNF therapies.
References
1. Costa L, et al. Drugs R D 2017;17:509–22.
2. Mease PJ, et al. Ann Rheum Dis. 2021;80:312–20.
To cite this abstract in AMA style:
Mease P, Luppino Assad R, Tsuji S, Richette P, Setty A, McDearmon-Blondell E, Gao T, Ciecinski S, Van den bosch F. Efficacy of Upadacitinib in Patients with Psoriatic Arthritis and Prior Exposure to Anti-TNF Therapy in the SELECT-PsA 2 Trial Through 2 Years [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-upadacitinib-in-patients-with-psoriatic-arthritis-and-prior-exposure-to-anti-tnf-therapy-in-the-select-psa-2-trial-through-2-years/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-upadacitinib-in-patients-with-psoriatic-arthritis-and-prior-exposure-to-anti-tnf-therapy-in-the-select-psa-2-trial-through-2-years/