Session Information
Date: Sunday, November 17, 2024
Title: Abstracts: Vasculitis – Non-ANCA-Associated & Related Disorders I: Clinical Trials
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Giant cell arteritis (GCA) is a chronic systemic vasculitis with limited targeted therapeutic options. The recent SELECT-GCA phase 3 trial demonstrated a favorable benefit-risk profile for upadacitinib 15 mg (UPA15), an oral selective JAK inhibitor, in treating patients with GCA.1 Here, we further analyzed the efficacy of UPA15 in various subgroups of patients categorized by baseline (BL) characteristics to better understand its efficacy in different patient subpopulations.
Methods: SELECT-GCA is a double-blind, placebo (PBO)-controlled phase 3 trial, in which patients randomly received UPA15 or upadacitinib 7.5 mg once daily in combination with a 26-week glucocorticoid (GC) taper regimen, or PBO with a 52-week GC taper regimen. The results of the primary analysis were previously reported.1 This subgroup analysis presents data from patients who received UPA15 or PBO. Eligible patients were aged ≥50 years with new-onset or relapsing GCA, who had active disease within 8 weeks of BL, received ≥ 40 mg prednisone (or equivalent) at any time before BL, and were taking ≥20 mg of prednisone (or equivalent) at BL. Achievement of the primary endpoint (sustained remission at week 52) and the secondary endpoint of sustained complete remission from week 12 through week 52 was descriptively evaluated in subgroups by BL demographic and disease characteristics. Sustained remission was defined as the absence of the signs or symptoms of GCA from week 12 through week 52 and adherence to the protocol-defined GC taper regimen. Sustained complete remission was defined as the achievement of sustained remission as well as normalization of both ESR and hsCRP from week 12 through week 52. Data were analyzed by nonresponder imputation with multiple imputation to handle missing data due to COVID-19 logistical restrictions.
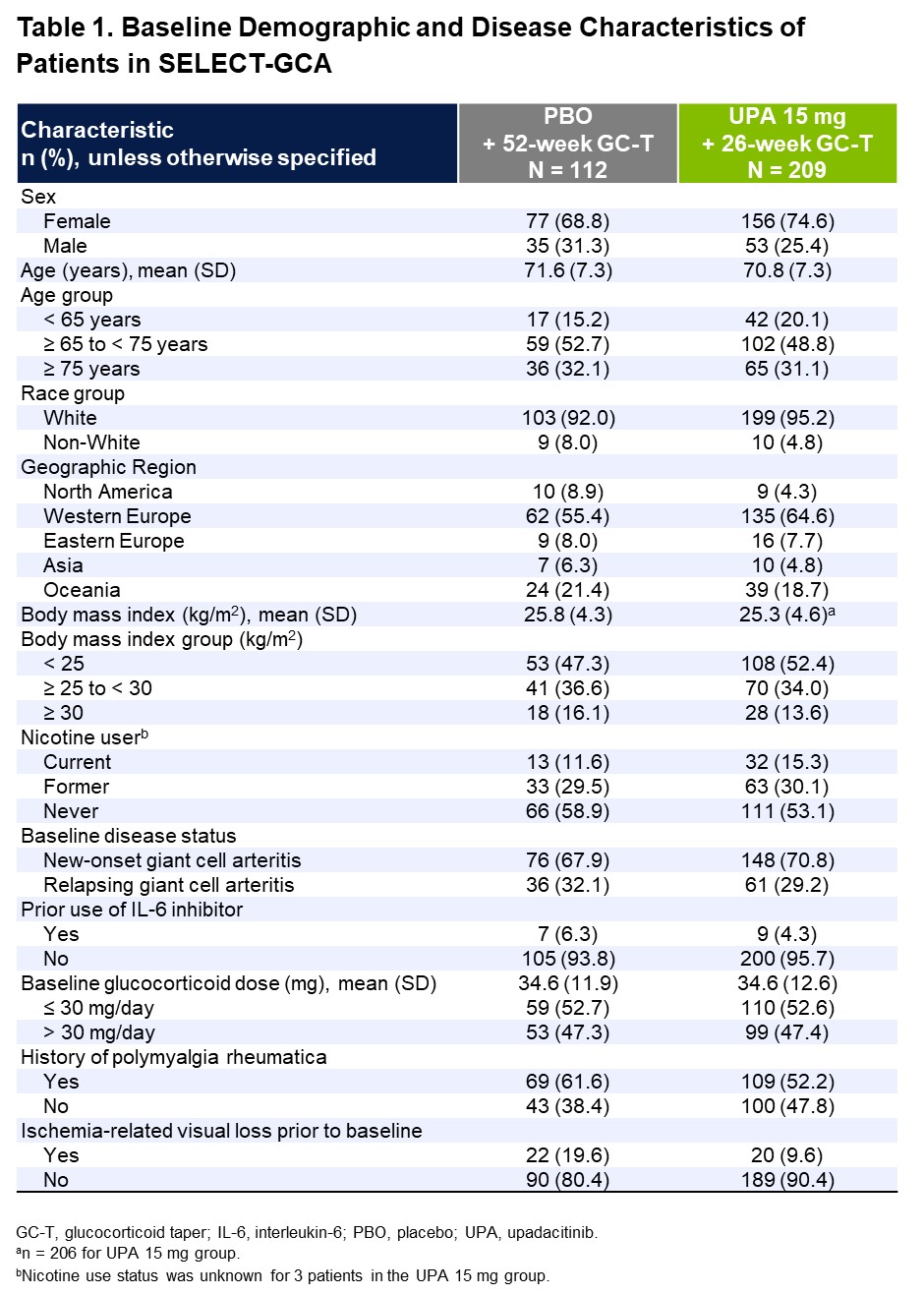
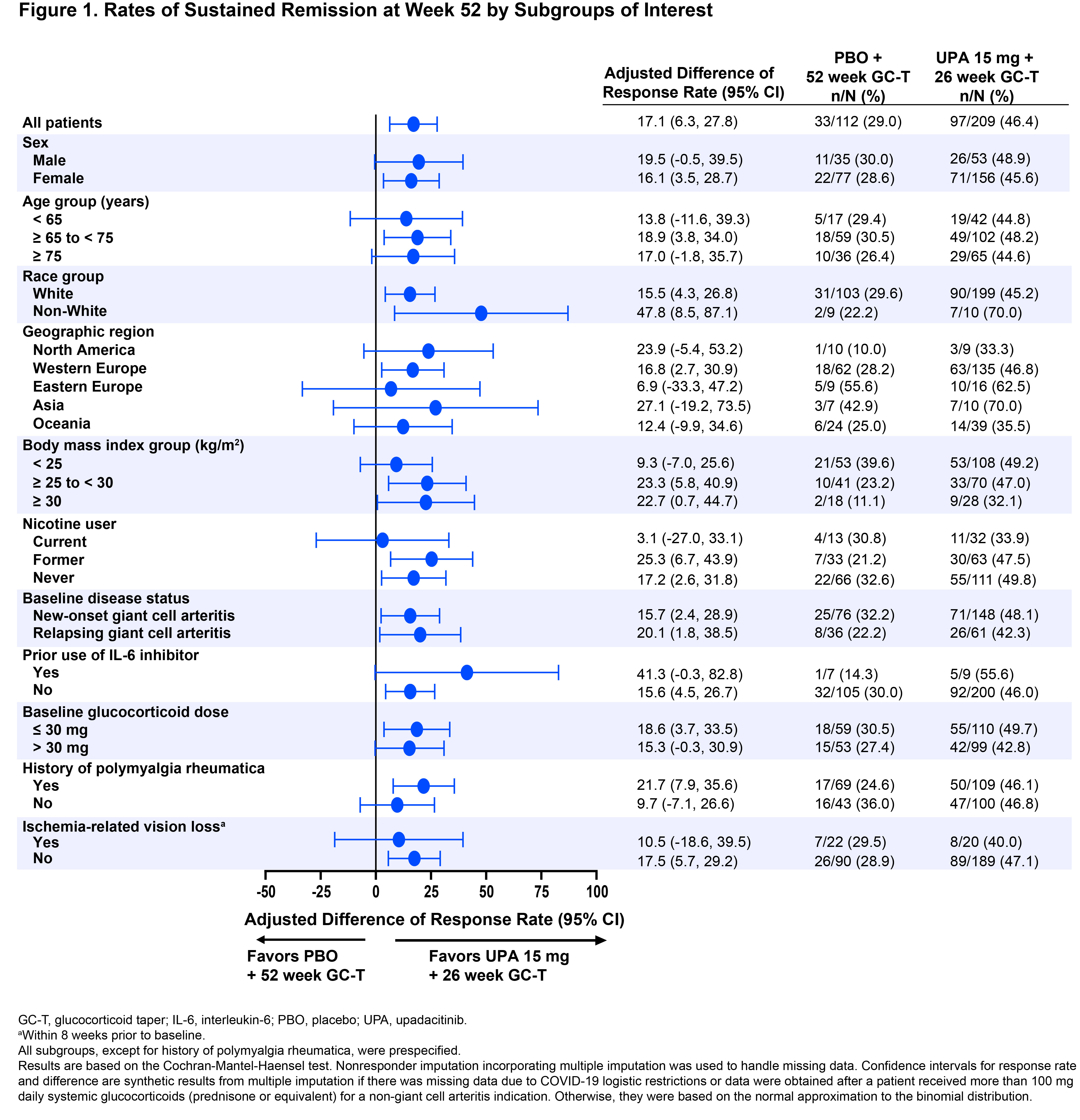
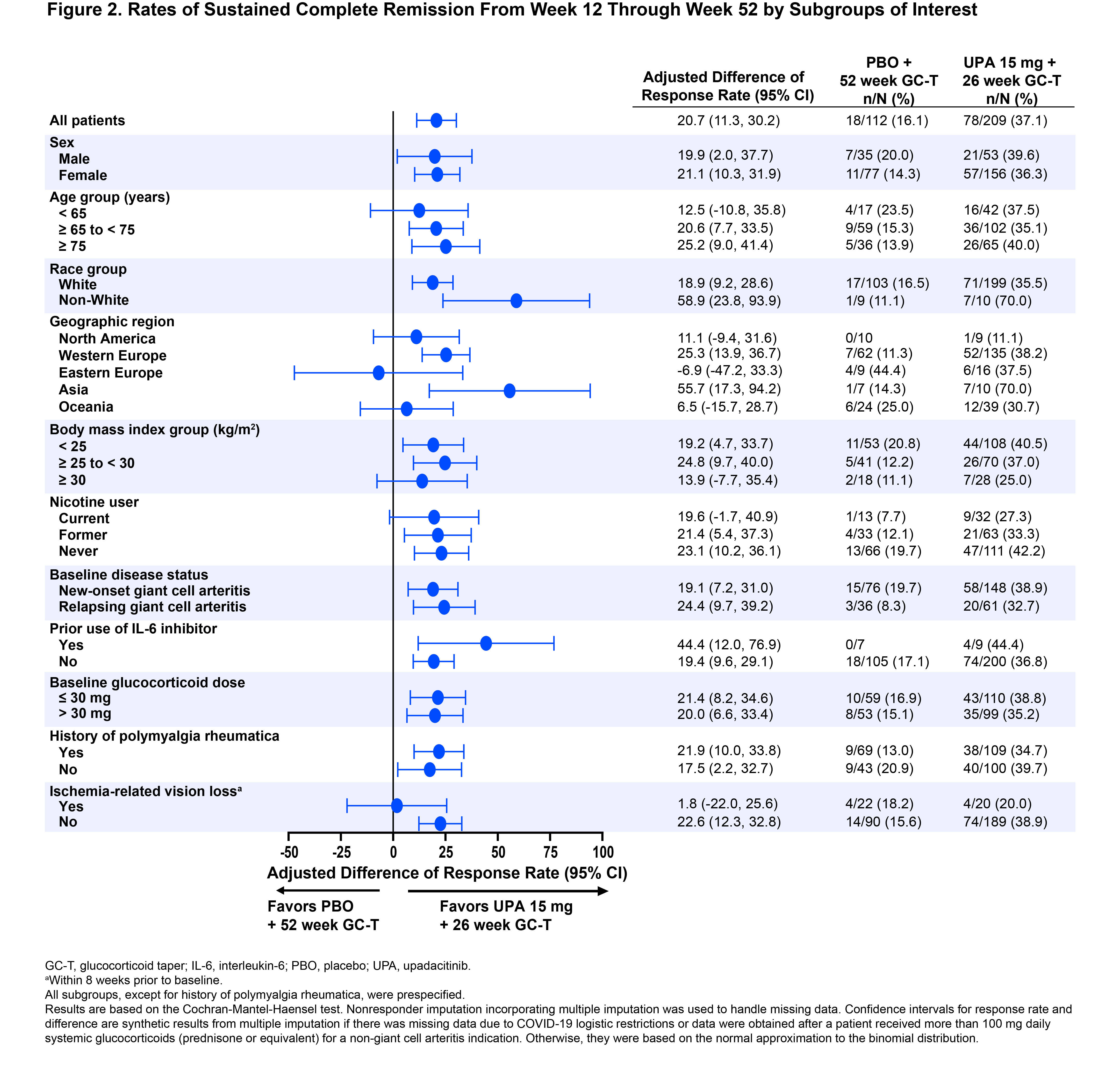
Results: A total of 321 patients were assigned and treated with UPA15 (N=209) or PBO (N=112). Patients’ BL characteristics were well balanced between the two treatment groups (Table 1): mean age was 71 years, 73% were female, 70% had new-onset GCA, and 30% had relapsing GCA. In the overall trial population, UPA15 demonstrated superior efficacy compared to PBO per the primary endpoint of sustained remission at week 52 (46% vs 29%, P=.0019) and sustained complete remission from week 12 through week 52 (37% vs 16%, P< .0001). Similarly, across nearly all subgroups analyzed, rates of sustained remission and sustained complete remission consistently favored UPA15 over PBO, including subgroups based on age, sex, new-onset vs relapsing GCA, history of polymyalgia rheumatica, and other BL characteristics (Figures 1 and 2). The response rates in some subgroups showed high variability, potentially due to limited sample sizes within those subgroups.
Conclusion: Across nearly all evaluated subgroups in SELECT-GCA, treatment with UPA15 resulted in generally similar rates of sustained remission and sustained complete remission as observed for the overall trial population. These results further support the efficacy of UPA15 across the population of patients with GCA.
References
1. Blockmans D, et al. Ann Rheum Dis. 2024;83(1):232.
To cite this abstract in AMA style:
Merkel P, Setty A, Blanco-Alonso R, Suppiah R, Daikeler T, Devauchelle V, Brouwer E, Tamaki H, Meng L, Yang Y, Joshi A, Phillips C, Wung P, Ponte C. Efficacy of Upadacitinib in Patients with Giant Cell Arteritis: Subgroup Analysis of the SELECT-GCA Phase 3 Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-upadacitinib-in-patients-with-giant-cell-arteritis-subgroup-analysis-of-the-select-gca-phase-3-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-upadacitinib-in-patients-with-giant-cell-arteritis-subgroup-analysis-of-the-select-gca-phase-3-trial/