Session Information
Date: Tuesday, November 9, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster III: Psoriatic Arthritis II (1801–1835)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
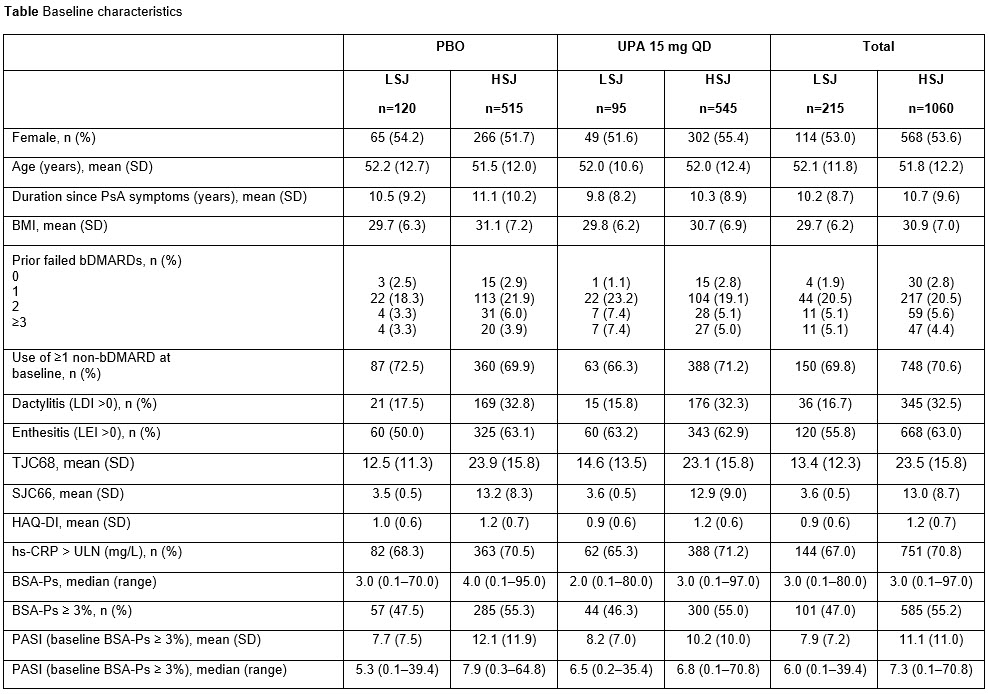
Background/Purpose: Although most patients with psoriatic arthritis (PsA) enrolled in clinical trials have polyarticular arthritis, patients in clinical practice may present with oligoarthritis. Data on the efficacy of Janus kinase inhibitors in patients with PsA with low joint counts are limited. The objective of this analysis was to evaluate the efficacy of upadacitinib (UPA) in subgroups of patients with PsA with a low (baseline swollen joint count [SJC] < 5) or high (SJC ≥5) SJC (LSJ or HSJ).
Methods: Data were pooled across the SELECT-PsA 11 (non-biologic disease-modifying antirheumatic drug [non-bDMARD] inadequate response [IR] or intolerance) and SELECT-PsA 22 (bDMARD IR or intolerance) trials, which both enrolled patients with ≥3 involved joints (SJC ≥3 and tender joint count [TJC] ≥3). Subgroup analysis was performed for patients with LSJ or HSJ treated with UPA 15 mg once daily (QD) or placebo (PBO). Efficacy endpoints included minimal disease activity (MDA), very low disease activity (VLDA), Psoriatic Arthritis Disease Activity Score (PASDAS) low disease activity (LDA; ≤3.2), PASDAS remission (≤1.9), and 20/50/70% improvement in American College of Rheumatology (ACR) criteria (ACR20/50/70), all at Week 24, and Psoriasis Area Severity Index (PASI) 75 and static Investigator Global Assessment of Psoriasis (sIGA) 0/1 at Week 16.
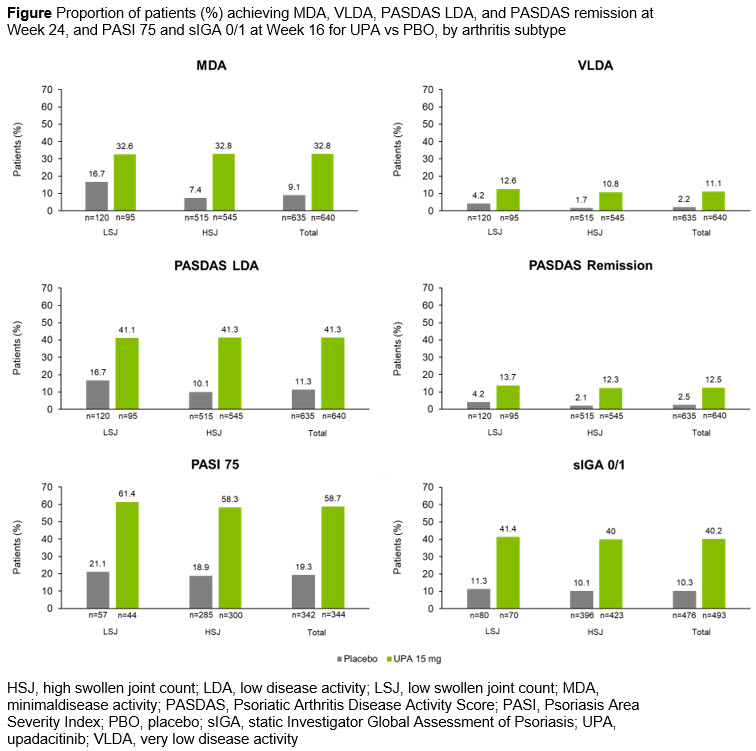
Results: At baseline, patients with HSJ (n=1060) had similar demographic characteristics but tended to have higher overall disease activity than patients with LSJ across multiple disease domains (n=215; Table). UPA efficacy appeared comparable in patients with LSJ and HSJ, with similar proportions of patients achieving composite (MDA, VLDA, PASDAS LDA, and PASDAS remission) measures at Week 24, and skin endpoints (PASI 75 and sIGA 0/1) at Week 16 (Figure). At Week 24, 60.0/36.8/22.1% of patients with LSJ receiving UPA 15 mg achieved ACR20/50/70 vs 40.0/17.5/5.8% in the PBO group; rates were 70.3/49.7/26.2% (UPA 15 mg) and 36.1/15.3/3.3% (PBO) in those with HSJ.
Conclusion: UPA efficacy was generally similar in patients with PsA with LSJ or HSJ, with both patient groups showing improvements in composite clinical endpoints and skin responses vs PBO.
References:
To cite this abstract in AMA style:
Gossec L, Gladman D, McDearmon-Blondell E, Sewerin P, Ritchlin C, Feng D, Lertratanakul A, Ranza R, Tam L, Marchesoni A, Coates L, Nash P. Efficacy of Upadacitinib in Patients with Active Psoriatic Arthritis and a Low or High Swollen Joint Count: A Subgroup Analysis of 2 Phase 3 Studies [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-upadacitinib-in-patients-with-active-psoriatic-arthritis-and-a-low-or-high-swollen-joint-count-a-subgroup-analysis-of-2-phase-3-studies/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-upadacitinib-in-patients-with-active-psoriatic-arthritis-and-a-low-or-high-swollen-joint-count-a-subgroup-analysis-of-2-phase-3-studies/