Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an oral JAK inhibitor for the treatment of RA. Greater improvements in efficacy outcomes have been reported with tofacitinib 5 mg BID ± conventional synthetic DMARDs in patients (pts) with early vs established RA.1,2 This post hoc analysis of ORAL Strategy data evaluated the efficacy and safety of tofacitinib monotherapy, tofacitinib + methotrexate (MTX), and adalimumab (ADA) + MTX, stratified by baseline (BL) RA duration.
Methods: ORAL Strategy (NCT02187055) was a P3b/4, 1-yr, double‑blind, triple‑dummy, active comparator-controlled study. MTX inadequate-responders were randomized 1:1:1 to receive tofacitinib 5 mg BID (tofa mono), tofacitinib 5 mg BID + MTX (tofa+MTX), or ADA SC 40 mg Q2W + MTX (ADA+MTX); MTX was dosed at 15–25 mg/wk except in cases of intolerance/toxicity. In this analysis, pts were stratified by BL RA duration as having early ( ≤ 2 yrs) or established ( > 2 yrs) RA. Efficacy outcomes (Months [M]3, 6 and 12): ACR20/50/70; change from BL (∆) in DAS28‑4(ESR), Simplified Disease Activity Index (SDAI) and Clinical Disease Activity Index (CDAI); and rates of DAS28-4(ESR)-, SDAI- and CDAI‑defined low disease activity ( ≤ 3.2, ≤ 11, and ≤ 10, respectively) and remission ( < 2.6, ≤ 3.3, and ≤ 2.8, respectively). Nominal p values for treatment comparisons were calculated without multiplicity adjustment; p < 0.05 was considered significant. Treatment-emergent AEs and serious AEs (SAEs) were assessed throughout the study.
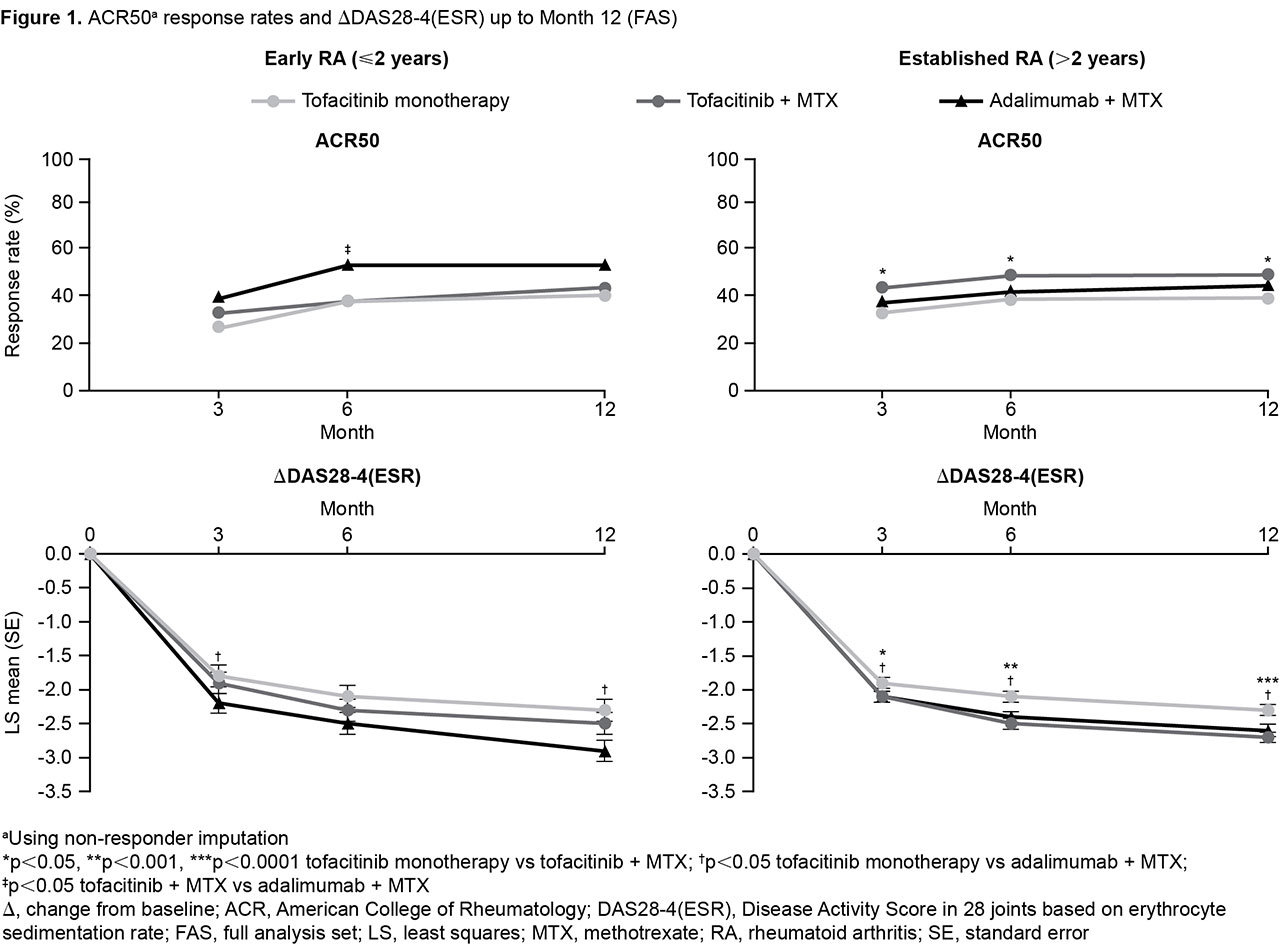
Results: In total, 241 pts had early RA (tofa mono: N=80; tofa+MTX: N=83; ADA+MTX: N=78; mean RA duration: 1.0–1.1 yrs); 905 pts had established RA (tofa mono: N=304; tofa+MTX: N=293; ADA+MTX: N=308; mean RA duration: 9.4–10.4 yrs). BL demographics and disease characteristics were generally comparable for early vs established RA pts, with some expected differences (the latter were slightly older, and a greater proportion had received prior biologic DMARDs); RF+ and anti-CCP+ rates were higher for established RA pts (Table 1). ACR50 and ∆DAS28-4(ESR) were generally similar for tofa mono and tofa+MTX up to M12 in early RA but significantly greater with tofa+MTX in established RA (p < 0.05); generally, ADA+MTX was not statistically different vs tofa+MTX in early RA (Figure 1). Trends were similar for all outcomes. AE rates were generally similar in early vs established RA. Trends for SAEs were less clear; rates were lowest for tofa+MTX in early RA and higher for both tofacitinib arms vs ADA+MTX in established RA (Table 1).
Conclusion: Efficacy was similar for tofa mono and tofa+MTX in early RA, and significantly higher with tofa+MTX in established RA. ADA+MTX was numerically but not always significantly more effective than tofacitinib in early RA. AE rates were generally similar regardless of BL RA duration. These findings, especially in established RA, are consistent with the conclusion of the primary analysis.3 Limitations to this post hoc analysis include low numbers of early RA pts.
- Hall S et al. Arthritis Rheum 2016; 68(S10): abstract 1609.
- Fleischmann RM et al. RMD Open 2016; 2: e000262.
- Fleischmann RM et al. Lancet 2017; 390: 457-468.
Acknowledgements: Study sponsored by Pfizer Inc. Medical writing support was provided by Christina Viegelmann of CMC Connect and funded by Pfizer Inc.
To cite this abstract in AMA style:
Takeuchi T, Tanaka Y, Sugiyama N, Iikuni N, Soma K, Shi H, Mysler E, Moots R, Smolen J, Fleischmann R. Efficacy of Tofacitinib Monotherapy, Tofacitinib with Methotrexate and Adalimumab with Methotrexate in Patients with Early ( ≤ 2 Years) vs Established ( > 2 Years) Rheumatoid Arthritis: A Post Hoc Analysis of Data from ORAL Strategy [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-tofacitinib-monotherapy-tofacitinib-with-methotrexate-and-adalimumab-with-methotrexate-in-patients-with-early-%e2%89%a4-2-years-vs-established-2-years-rheumatoid-arthritis-a-p/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-tofacitinib-monotherapy-tofacitinib-with-methotrexate-and-adalimumab-with-methotrexate-in-patients-with-early-%e2%89%a4-2-years-vs-established-2-years-rheumatoid-arthritis-a-p/