Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor under investigation for psoriatic arthritis (PsA). The efficacy of tofacitinib has been evaluated in 2 Phase 3 studies in patients (pts) with PsA. In this analysis, we describe the efficacy of tofacitinib by background methotrexate (MTX) dose in pts with PsA.
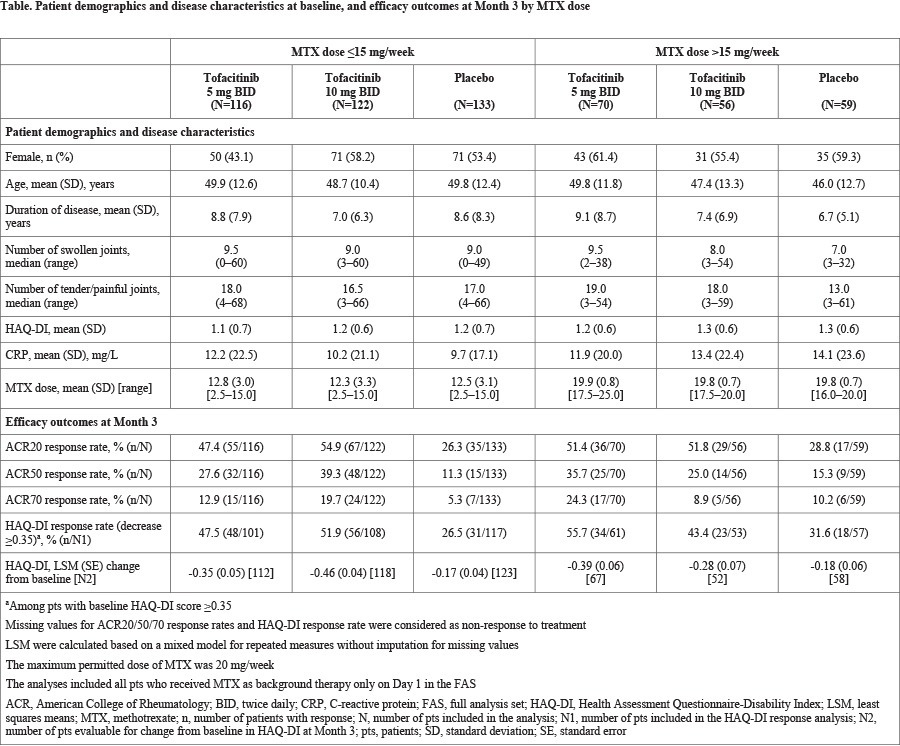
Methods: This post hoc analysis utilized efficacy data pooled from 2 Phase 3, randomized, double-blind, placebo-controlled studies (OPAL Broaden [12 months; NCT01877668] and OPAL Beyond [6 months; NCT01882439]) in pts with a diagnosis (≥6 months) of active PsA (≥3 swollen and ≥3 tender joints). Pts in OPAL Broaden were tumor necrosis factor inhibitor (TNFi)-naïve and had an inadequate response (IR) to ≥1 conventional synthetic disease-modifying antirheumatic drug (csDMARD). Pts in OPAL Beyond had an IR to ≥1 TNFi. Pts were randomized to tofacitinib 5 or 10 mg twice daily (BID), placebo, or adalimumab 40 mg subcutaneous every 2 weeks (OPAL Broaden; adalimumab data not shown). All pts received a stable dose of 1 csDMARD (eg MTX, leflunomide, or sulfasalazine) as background therapy. The maximum dose of MTX allowed per protocol was 20 mg/week. Efficacy outcomes for tofacitinib at Month 3 were evaluated by background MTX dose (≤15 vs >15 mg/week) and included: ACR20/50/70 response rates (≥20/50/70% improvement from baseline, respectively), Health Assessment Questionnaire-Disability Index (HAQ-DI) response rate (reduction from baseline ≥0.35 points), and mean change from baseline in HAQ-DI score. Analyses were based on the full analysis set for pts receiving MTX on Day 1; pts with missing data were considered as having a non-response for binary endpoints. No statistical testing was performed.
Results: In total, data from 556 pts who received tofacitinib plus MTX only or placebo plus MTX only (tofacitinib 5 mg BID, n=186; tofacitinib 10 mg BID, n=178; placebo, n=192) were included in this analysis. Most pts were treated with background MTX at doses ≤15 mg/week (n=371, 66.7%; mean [SD] dose, 12.6 [3.1] mg/week) vs >15 mg/week (n=185, 33.3%; mean [SD] dose, 19.8 [0.8] mg/week). Baseline demographics and disease characteristics were generally similar between arms in MTX dose groups (Table). At Month 3, tofacitinib 5 and 10 mg BID were generally associated with numerically greater ACR and HAQ-DI response rates and greater changes from baseline in HAQ-DI score compared with placebo. The magnitude of tofacitinib effects on efficacy outcomes appeared broadly similar between background MTX dose groups (Table).
Conclusion: The results of this pooled analysis suggest that the efficacy of tofacitinib in pts with PsA was greater than placebo and does not differ when evaluated by background MTX dose (≤15 vs >15 mg/week), although small pt numbers in some groups may limit the conclusions that can be made. These results are consistent with findings from similar analyses of tofacitinib in pts with rheumatoid arthritis.
To cite this abstract in AMA style:
Kivitz AJ, FitzGerald O, Nash P, Pang S, Azevedo VF, Kudlacz E, Wang C, Graham D, Takiya L. Efficacy of Tofacitinib By Background Methotrexate Dose in Patients with Psoriatic Arthritis: A Post Hoc Analysis of Pooled Data from 2 Phase 3 Trials [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-tofacitinib-by-background-methotrexate-dose-in-patients-with-psoriatic-arthritis-a-post-hoc-analysis-of-pooled-data-from-2-phase-3-trials/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-tofacitinib-by-background-methotrexate-dose-in-patients-with-psoriatic-arthritis-a-post-hoc-analysis-of-pooled-data-from-2-phase-3-trials/