Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Tildrakizumab (TIL), an anti–interleukin-23p19 monoclonal antibody, is approved in the US, EU, and Australia for treatment of moderate to severe plaque psoriasis. A randomized, double-blind, placebo-controlled, multiple-dose, phase 2b study (NCT02980692) evaluating efficacy and safety of TIL for treatment of psoriatic arthritis (PsA) was recently completed. Treating to a target of remission or low disease activity has been recommended for patients with PsA.
Methods: Patients (pts) ≥18 years old with PsA2 and ≥3 tender and ≥3 swollen joints were randomized 1:1:1:1:1 to receive TIL (200 mg once every 4 weeks [Q4W], 200 mg every 12 weeks [Q12W], 100 mg Q12W, or 20 mg Q12W) or placebo (PBO Q4W) to W24. Thereafter, PBO Q4W and TIL 20 mg Q12W arms crossed over to TIL 200 mg Q12W to W52. Post hoc analyses were performed to evaluate the proportion of pts with Psoriatic Arthritis Disease Activity Score (PASDAS) < 3.2 (low disease activity), and remission in Disease Activity in Psoriatic Arthritis (DAPSA) (defined as scores between 0–4). Adverse events (AEs), including treatment-emergent AEs (TEAEs) and serious AEs (SAEs), were monitored throughout the study. Because this was a post-hoc analysis, no statistical analyses were performed to compare tildrakizumab groups to placebo.
Results: Overall, 391/500 pts screened met the inclusion criteria; demographics and baseline disease characteristics were comparable between treatment groups.
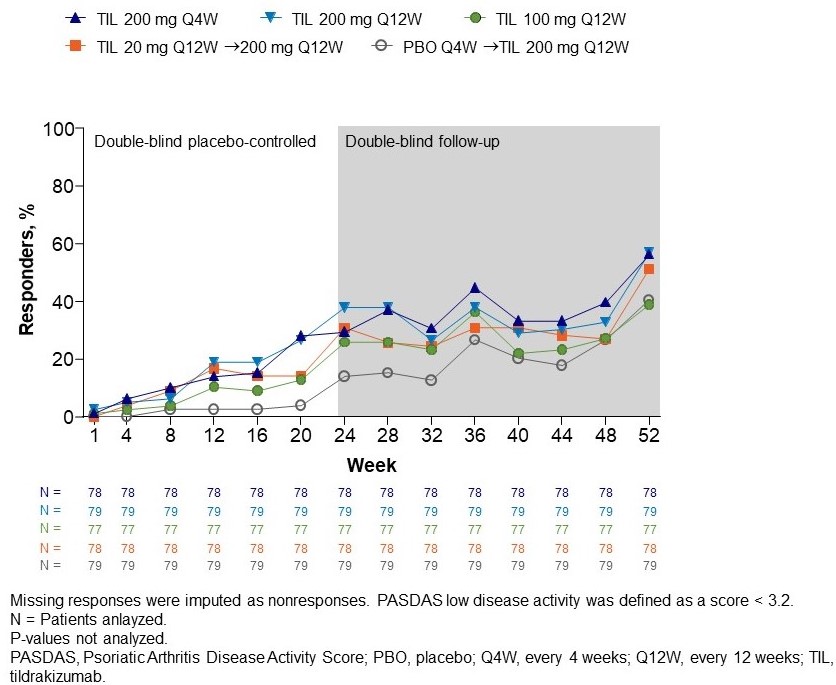
At 24 weeks, a numerically greater proportion of patients in the TIL vs PBO arms achieved DAPSA remission, with the proportion of patients in remission increasing thereafter to week 52 (Figure 1). At week 24, there was a numerically greater proportion of patients in the TIL vs placebo arms with low disease activity in PASDAS < 3.2. The proportion of patients with PASDAS < 3.2 continued to increase up to week 52 (Figure 2).
From W0–W52, 64.5% and 3.3% of pts experienced a TEAE and SAE, respectively. The most common TEAEs were nasopharyngitis (8.4%) and upper respiratory tract infection (6.4%). Two (0.5%) patients had a fungal skin infection (candida, tildrakizumab 200 mg Q4W arm). Most TEAEs, including infections, were mild. No deaths were reported.
Conclusion: PsA pts treated with TIL achieved desirable low disease activity and remission targets of treatment. TIL was well tolerated among patients in all groups through 52 weeks of treatment.
References:
1) Reich K, et al. Lancet. 2017;390(10091):276−88.
2) Taylor W, et al. Arthritis Rheum. 2006; 54(8):2665−73.
 Figure 1. Proportion of patients in remission based on DAPSA
Figure 1. Proportion of patients in remission based on DAPSA
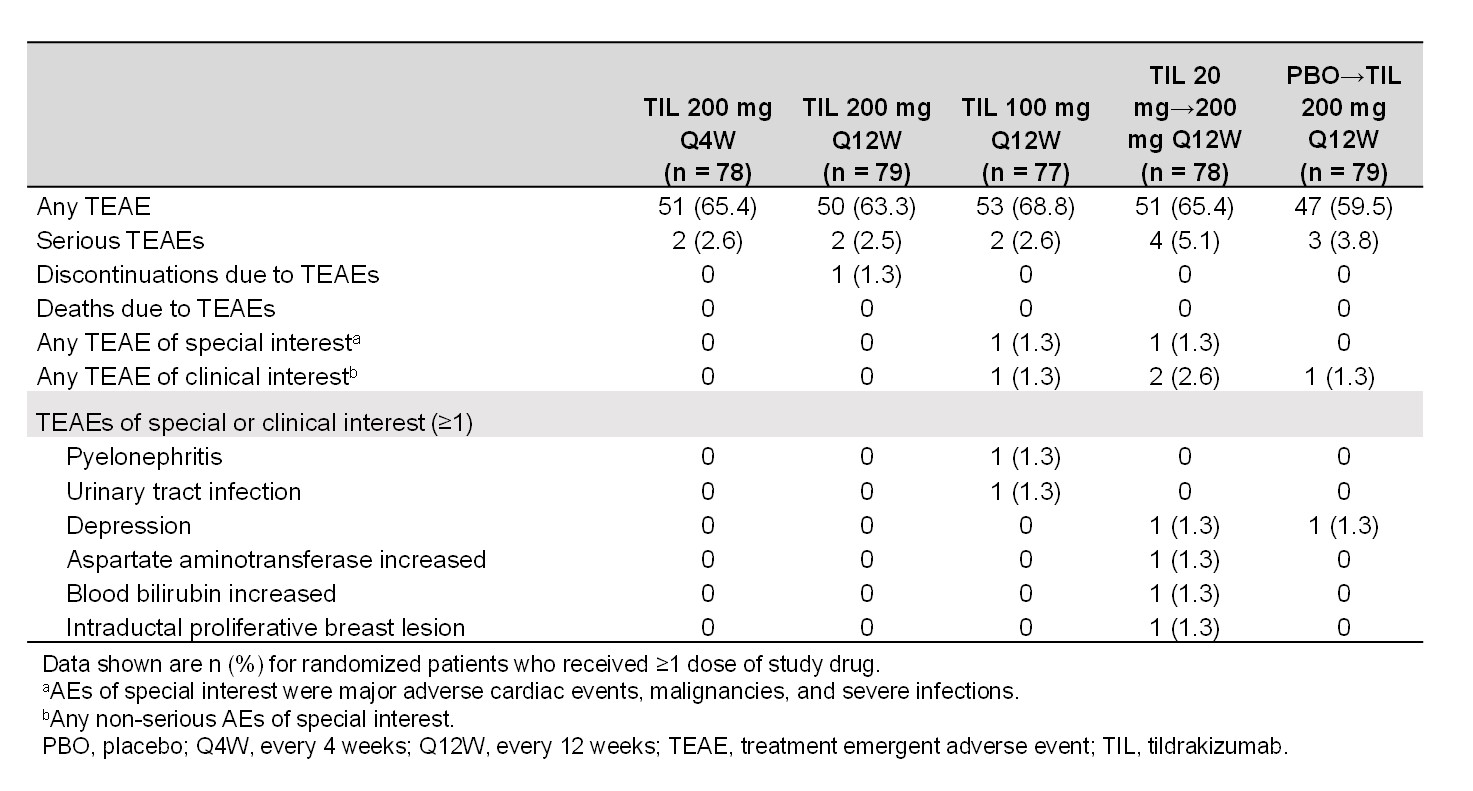 Table 1. Summary of safety findings to week 52
Table 1. Summary of safety findings to week 52
To cite this abstract in AMA style:
Chohan S, Kavanaugh A, Strand V, Chou R, Mendelsohn A, Rozzo S, Mease P. Efficacy of Tildrakizumab in PsA: DAPSA Remission and Low Disease Activity in PASDAS Through Week 52 [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-tildrakizumab-in-psa-dapsa-remission-and-low-disease-activity-in-pasdas-through-week-52/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-tildrakizumab-in-psa-dapsa-remission-and-low-disease-activity-in-pasdas-through-week-52/