Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Tyrosine kinase 2 (TYK2) mediates signaling of key cytokines involved in plaque psoriasis (PsO) and PsA pathophysiology. Deucravacitinib is a first-in-class, oral, selective, allosteric TYK2 inhibitor approved in multiple countries for the treatment of adults with moderate to severe PsO, based on superiority of deucravacitinib over apremilast and placebo (PBO) in various outcome measures in 4 phase 3 trials, including in China and Japan (NCT03611751, NCT03624127, NCT04167462, NCT03924427) in patients with moderate to severe PsO.1,2 DEUC also improved multiple measures of disease activity, including in arthritis and PsO, vs PBO in a phase 2 trial (NCT03881059) in patients with active PsA.3 This analysis further evaluated the efficacy ofdeucravacitinib on PsO in patients with concomitant PsA in the phase 2 trial.
Methods: The phase 2 double-blind trial in PsA randomized patients (N=203) 1:1:1 to PBO, deucravacitinib 6 mg once daily (QD), or deucravacitinib 12 mg QD. After wk 16 (part A), patients could optionally enroll in a double-blind period until wk 52 (part B), where deucravacitinib-treated patients with minimal disease activity at wk 16 continued deucravacitinib treatment to wk 52. PsO disease activity measures, including mean body surface area (BSA), mean Psoriasis Area and Severity Index (PASI) score, and achievement of different treat-to-target PASI and BSA thresholds, were assessed at week 16 in part A.
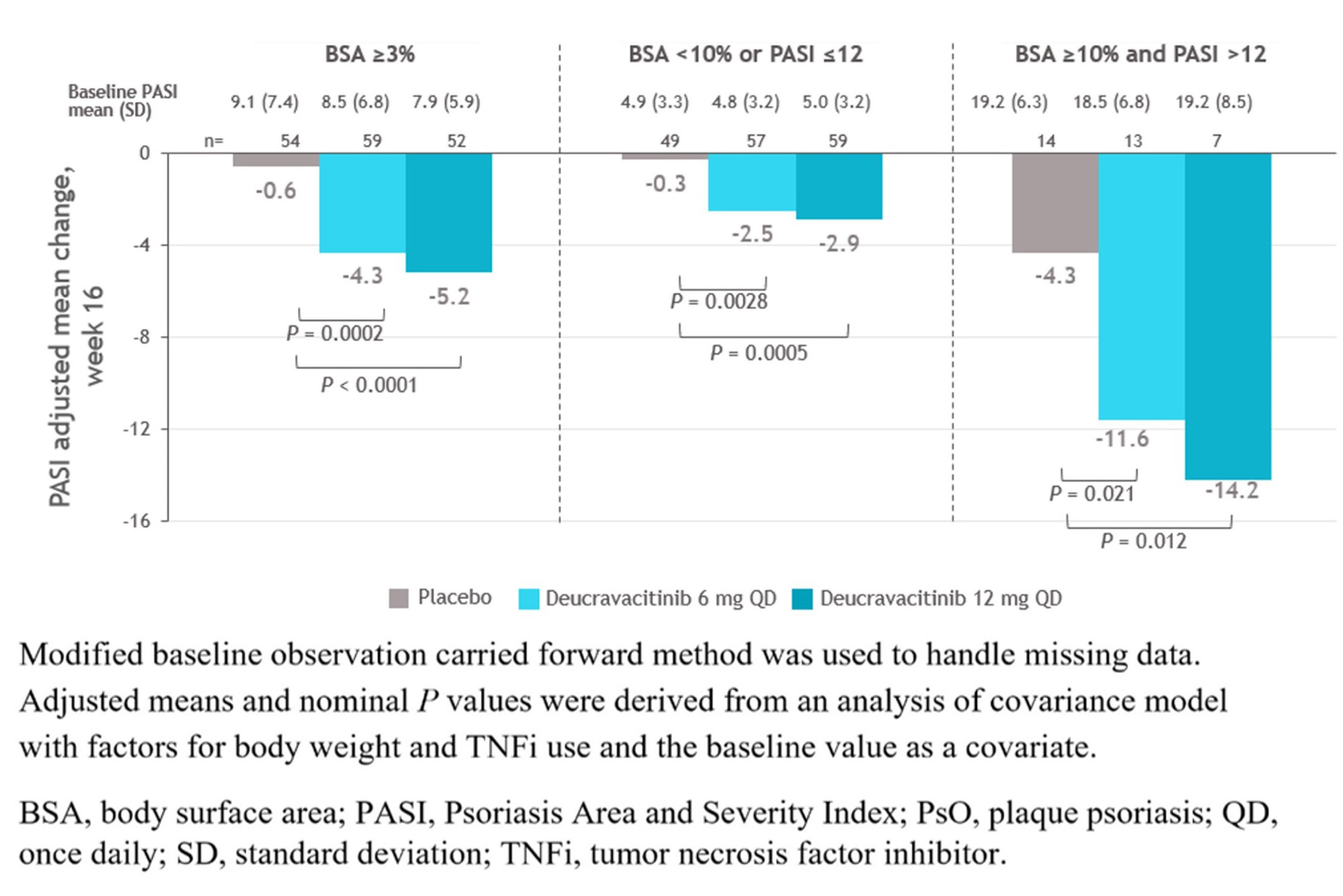
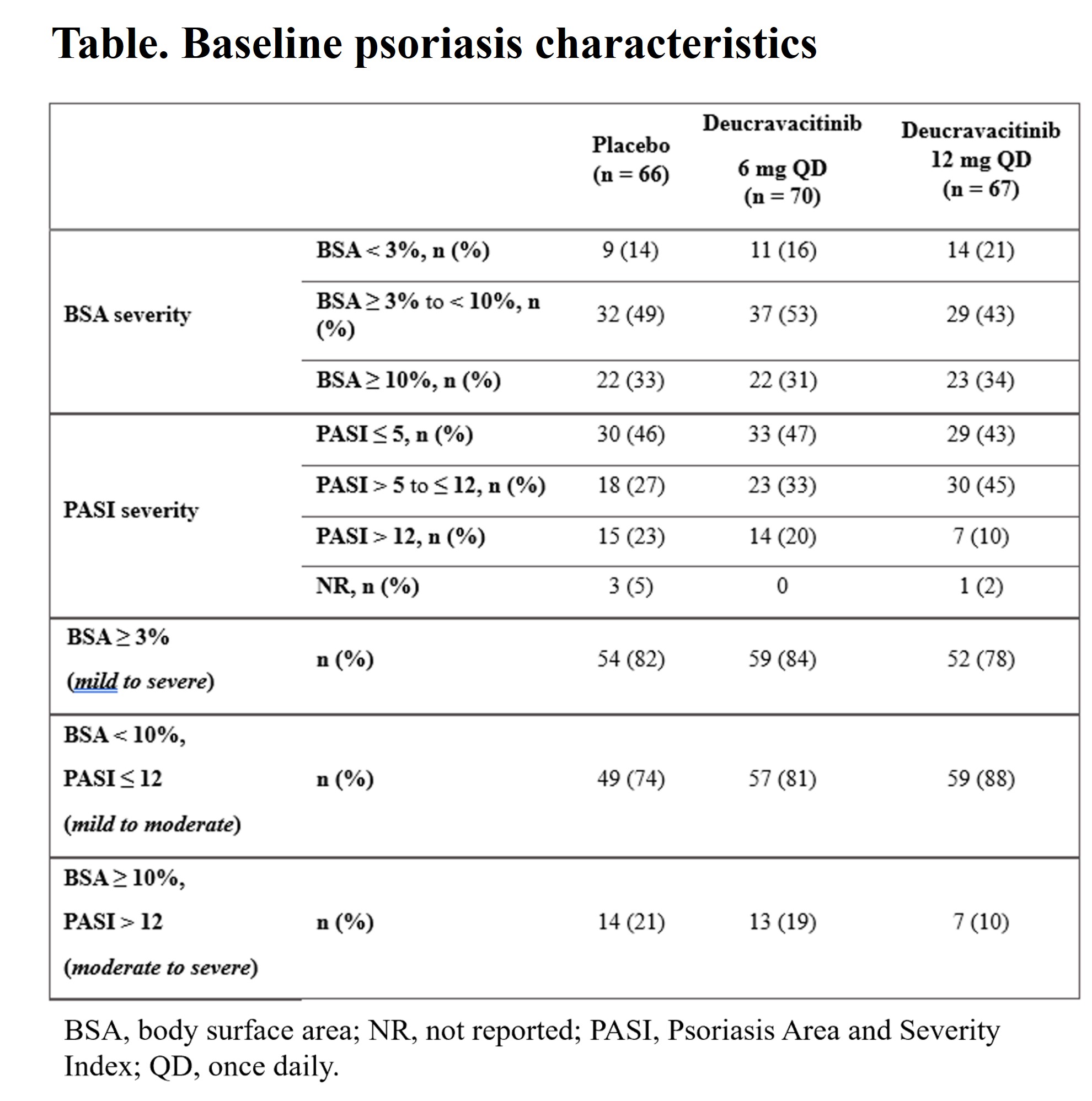
Results: At baseline (BL), PsO characteristics were generally similar across treatment groups, with most evaluable patients (≥ 74%) having a PASI< 12 or BSA< 10 (Table). At wk 16, mean PASI scores significantly decreased from BL and the improvements were significantly greater with deucravacitinib in PBO in patients treated with deucravacitinib vs PBO, even in those with very low BL PASI scores (Figure). Deucravacitinib treatment significantly decreased PASI scores from BL in patients with 6 mg QD and 12 mg QD vs PBO, respectively inpatients on background conventional synthetic (cs) DMARD (−4.0 and −4.9 vs −2.3; P < 0.05 for both) as well as those without csDMARD use (−3.7 and −4.0 vs +2.5; P < 0.001, for both) vs PBO. At wk 16, a greater proportion of patients treated with deucravacitinib at either dose vs PBO achieved PASI ≤ 1 in patients with mild to moderate or moderate to severe PsO. Decreases in mean PASI score at wk 16 were maintained through wk 52 in patients who achieved MDA and continued treatment with deucravacitinib 6 mg or 12 mg QD in part B of the study.
Conclusion: Treatment with deucravacitinib significantly improved PsO in patients with PsA, regardless of BL PsO severity and background csDMARD use. Of note, improvement in the moderate to severe PsO patient subgroup was comparable with that observed in the phase 3 POETYK PSO-1 trial in patients with moderate to severe PsO.1
References:
1. Armstrong A, et al. J Am Acad Dermatol 2023;88:29–39.
2. Strober B, et al. J Am Acad Dermatol 2023;88:40–51.
3. Mease PJ, et al. Ann Rheum Dis 2022;81:815–822.
To cite this abstract in AMA style:
Gottlieb A, Armstrong A, Merola J, Napoli A, Nowak M, Banerjee S, Lehman T, Mease P. Efficacy of the Oral, Selective, Allosteric Tyrosine Kinase 2 Inhibitor, Deucravacitinib, on Psoriasis in Patients with Active PsA: Results from a Phase 2 Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-the-oral-selective-allosteric-tyrosine-kinase-2-inhibitor-deucravacitinib-on-psoriasis-in-patients-with-active-psa-results-from-a-phase-2-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-the-oral-selective-allosteric-tyrosine-kinase-2-inhibitor-deucravacitinib-on-psoriasis-in-patients-with-active-psa-results-from-a-phase-2-trial/