Session Information
Date: Monday, November 6, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Approximately 30-50% of patients with axial SpA (axSpA) who receive a first bDMARD do not respond well. Current practice in these patients is switching to another bDMARD but the scientific evidence for this attitude is sparse. The aims of this study were: First, to investigate if switching biological DMARDs (bDMARDs) after the failure to prior bDMARD is efficacious in patients with axSpA. Secondly, to evaluate the influence on this efficacy of: i) the reason to discontinue prior TNF inhibitor (TNFi), ii) changing the type of TNFi and iii) changing the target.
Methods: A systematic literature review until January 2017 was performed using Medline, EMBASE and Cochrane databases. The research question was formulated according to the PICOS method: Population (axSpA patients with failure to at least one bDMARD); Intervention (second or consecutive bDMARD); Outcome (clinical response according to ASAS, BASDAI or ASDAS criteria); and Study design (longitudinal studies with at least 50 patients and 12 weeks of follow-up). Data were extracted by two independent reviewers using a specific form. Results are shown as relative frequencies (%).
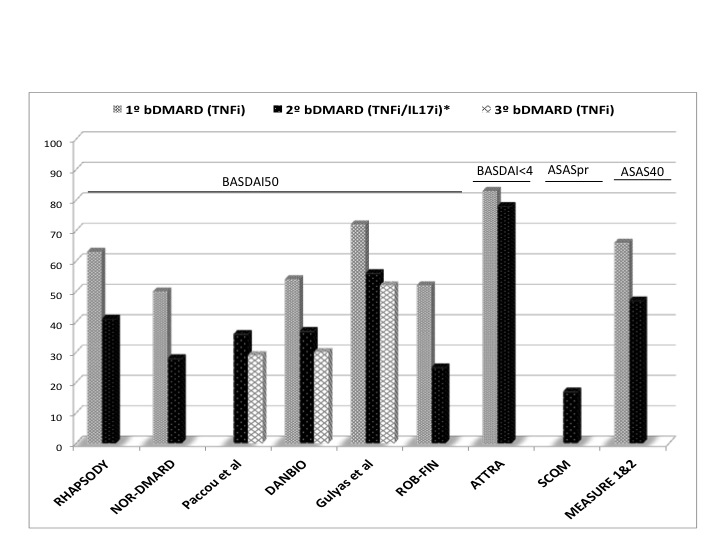
Results: In total, 9 studies out of 1,862 citations were included. Most of them were observational studies reporting data from national registries and only two used data from clinical trials. In these studies, 1,956 patients (91% AS and 9% nr-axSpA) switched to a second bDMARD (97% TNFi and 3% IL-17i) and 170 to a third bDMARD (all TNFi). The most common reason was inefficacy, followed by intolerance or adverse events related to the administered drug. Baseline characteristics (median -range-) of patients who switched bDMARDs were: age 43 (38-46) years old, 67% (54-80) males, 77% (62-84) HLA-B27+, and BASDAI before switching drugs 6.2 (5.2-7.1). As a control group, data from 4,191 patients after receiving the first bDMARD were analysed.
Time to assess response after switching was 6 (3-12) months. The criteria to define clinical response were heterogeneous. Clinical response (BASDAI50) after a second TNFi was achieved by 25-56% of patients compared to 50-72% after the first TNFi. Also, 47% of patients switching to IL-17i after a TNFi responded (ASAS40) compared to 66% in those who received IL-17i as first line (Figure). The response after switching was not influenced by the reason to discontinue, type of prior TNFi or changing the target.
Conclusion: In patients with axSpA, switching to a second bDMARD (a TNFi or IL-17i) after prior TNFi is efficacious. Nevertheless, the clinical response is lower than the observed in patients naive to bDMARD. So far, the reason to discontinue prior bDMARD or the type of bDMARD have not been identified as predictors of response. Published evidence for switching to a third bDMARD is lacking.
Figure: Efficacy as first, second and third bDMARD in patients with axial spondyloarthritis.
*IL-17i only in MEASURE1&2 study.
To cite this abstract in AMA style:
Navarro-Compán V, Plasencia-Rodriguez C, De Miguel E, Diaz del Campo P, Balsa A, Gratacos-Masmitja J. Efficacy of Switching Biological Dmards in Patients with Axial Spondyloarthritis: Results from a Systematic Literature Review [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-switching-biological-dmards-in-patients-with-axial-spondyloarthritis-results-from-a-systematic-literature-review/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-switching-biological-dmards-in-patients-with-axial-spondyloarthritis-results-from-a-systematic-literature-review/