Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: A global, phase 3 study (SIRROUND-T) evaluating the efficacy and safety of sirukumab, a selective, high-affinity human monoclonal antibody to IL-6, has recently been conducted in patients (pts) with active rheumatoid arthritis (RA) despite anti-TNF therapy. This post hoc analysis sought to compare the efficacy of sirukumab in subgroups of pts with active RA refractory to anti-TNF treatment who previously also received non-anti-TNF biologic therapy (TNFs + other biologics) and who did not previously receive other biologic therapy, other than anti-TNF biologic therapy (TNF only).
Methods: Pts were randomized in a 1:1:1 ratio to sirukumab subcutaneous (SC) 50 mg q4w, sirukumab SC 100 mg q2w, or placebo SC q2w. Pts on placebo with insufficient (<20%) improvement at Wk 18 or still on placebo at Wk 24 were re-randomized to 1 of the 2 sirukumab doses. The primary efficacy endpoint was ACR20 response at Wk 16, and major secondary endpoints included change from baseline (BL) in HAQ-DI score at Wk 24, ACR50 response at Wk 24, and DAS28 (CRP) remission at Wk 24. This post-hoc analysis compared efficacy for TNF only and TNF + biologics pts for sirukumab versus placebo, and across categories within sirukumab dose groups.
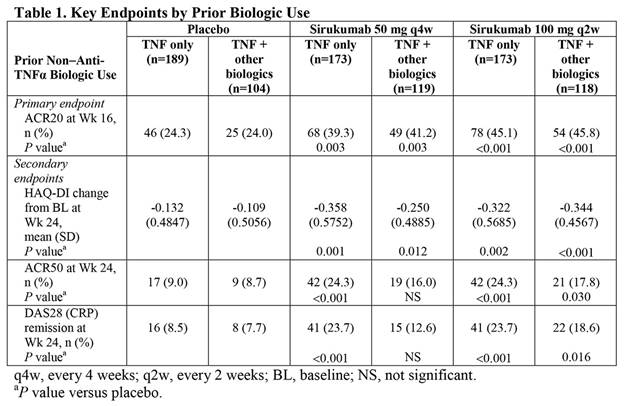
Results: In the placebo, sirukumab 50 mg q4w, and sirukumab 100 mg q2w groups, respectively, 104, 119, and 118 pts had previously received non-anti-TNF biologic therapy, in addition to prior TNF therapy. Improvements were observed in all outcomes for sirukumab versus placebo, regardless of prior biologic use (Table 1). Significantly more pts achieved ACR20 at Wk 16 with sirukumab 50 mg q4w and 100 mg q2w compared with placebo (all P £0.003). Both doses of sirukumab led to significantly greater improvements in HAQ-DI change from BL at Wk 24 than placebo (all P £0.012). Significantly more pts (TNF only at both sirukumab doses and TNF + biologics taking sirukumab 100 mg q2w) achieved ACR50 and DAS28 (CRP) remission at Wk 24 with sirukumab treatment compared with placebo; TNF + biologics pts on sirukumab 50 mg q4w had only numerically higher rates of ACR50 and DAS28 (CRP) remission at Wk 24 compared with placebo.
Conclusion: In general, comparable efficacy was observed with both sirukumab doses between pts with active RA despite anti-TNF therapy who had received TNFs only and TNFs + other biologics.
To cite this abstract in AMA style:
Tanaka Y, Aletaha D, Bingham C III, Agarwal P, Kurrasch R, Popik S. Efficacy of Sirukumab, an Anti–IL-6 Cytokine Monoclonal Antibody, Based upon Prior Use of Non-Anti-TNF Biologics in Patients with Active Rheumatoid Arthritis Despite Anti-TNF Therapy: Results from a Global Phase 3 Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-sirukumab-an-anti-il-6-cytokine-monoclonal-antibody-based-upon-prior-use-of-non-anti-tnf-biologics-in-patients-with-active-rheumatoid-arthritis-despite-anti-tnf-therapy-results/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-sirukumab-an-anti-il-6-cytokine-monoclonal-antibody-based-upon-prior-use-of-non-anti-tnf-biologics-in-patients-with-active-rheumatoid-arthritis-despite-anti-tnf-therapy-results/