Session Information
Date: Sunday, November 7, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster I: Axial Spondyloarthritis (0908–0939)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Human Leukocyte Antigen (HLA)-B is strongly associated with axial spondyloarthritis (axSpA); over 100 subtypes of HLA-B27 are currently recognized and designated as HLA-B∗2701 to HLA-B∗27106, defined by their DNA sequence.1,2 The association of these subtypes with the clinical features of axSpA patients or their response to therapy has not been determined so far. In this post hoc analysis, we explored the potential association of the HLA-B27 subtypes with the effect of secukinumab (SEC) in axSpA patients from the SKIPPAIN trial (NCT03136861).
Methods: SKIPPAIN, a 24 week (wk), randomised, double-blind, placebo (PBO) controlled, multicenter trial, enrolled adult axSpA patients with active disease fulfilling ASAS classification criteria (Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] score ≥4 and average spinal pain numerical rating scale [NRS] score >4 at baseline [BL]) and inadequate response to ≥2 NSAIDs for ≥4 wks. Patients were randomized (3:1) to receive subcutaneous SEC 150 mg or PBO wkly followed by every 4 wks (q4w) starting at Wk 4. At Wk 8, PBO patients were re-randomized to SEC 150 or 300 mg q4w up to Wk 24. HLA-B27 subtypes were tested by PCR-reverse sequence-specific oligonucleotide probe on a baseline blood sample. Average spinal pain scores were analysed using a repeated measures analysis of covariance (ANOVA) model.
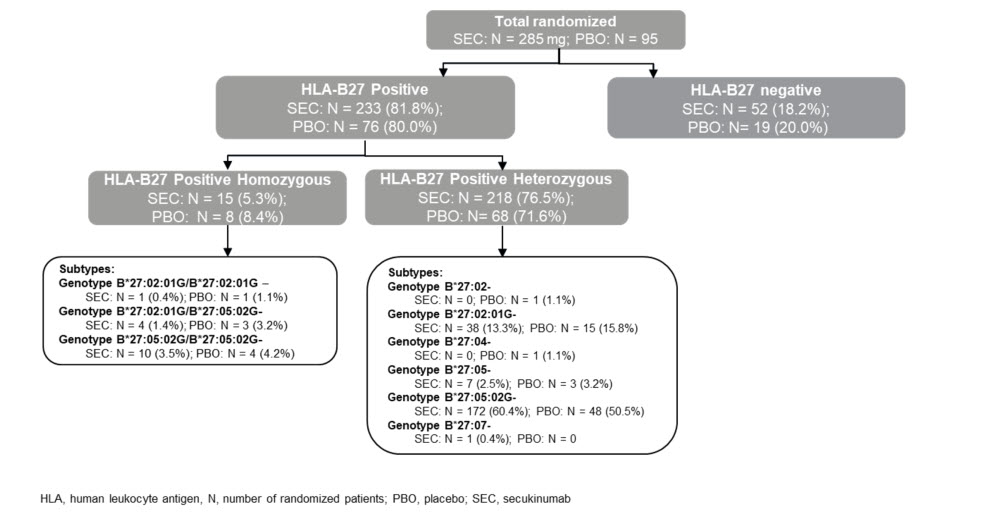
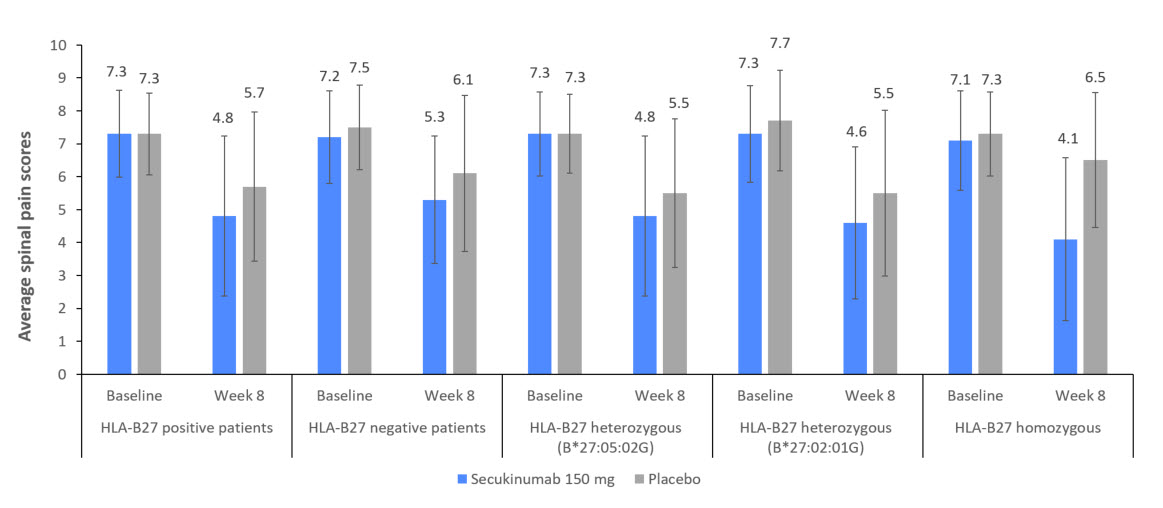
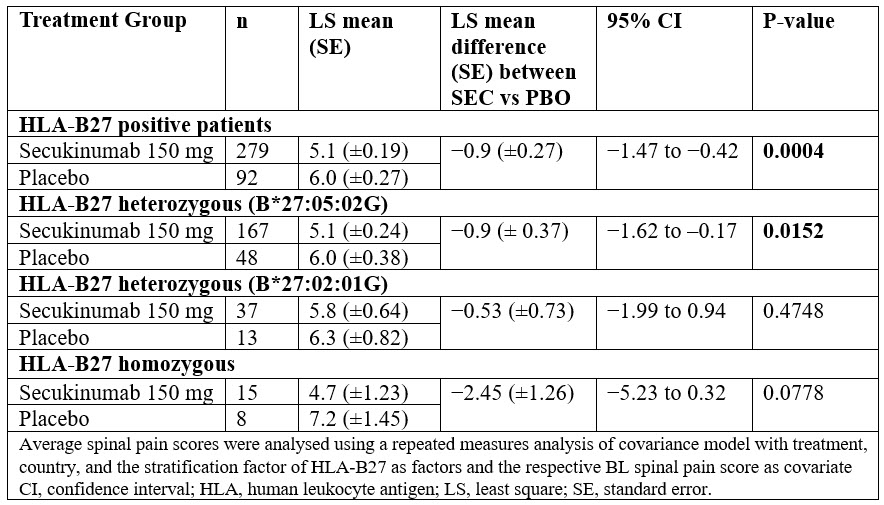
Results: Overall 380 patients with axSpA (269 [70.8%] AS and 111 [29.2%] nr-axSpA) were randomised to SEC 150 mg (N=285) or PBO (N=95). Demographic and BL characteristics were previously reported.3 Most patients enrolled in the study were HLA-B27 positive in both treatment groups (233 [81.8%] in the SEC vs. 76 [80.0%] in the PBO group). The distribution of HLA subtypes was consistent with what is expected in a typical European population1, with HLA-B*27:05:02G as the most common allele (Figure 1). In the HLA-B*27 homozygous group, a higher proportion of patients had uveitis, peripheral arthritis, enthesitis and a family history of spondyloarthritis, when compared to the heterozygous group; disease severity or burden of disease was similar between the two groups. Male predominance was more evident in the HLA-B*27 homozygous group who were younger by one year and reported longer duration of symptoms by an average of 8 months. Disease severity was comparable while disease burden was higher in B*27:05:02G compared with B27:02:01G heterozygous group. There were notable differences in the back pain response associated with B27 subtypes although the level of statistical significance could not always be reached due to the size of the subgroups (Figure 2 and Table).
Conclusion: The spinal pain response after 8 weeks of treatment showed notable differences between HLA-B27 subgroups. Further studies or pooled analyses are warranted to investigate associations of the HLA-B27 subtypes with the clinical features of axSpA or of less prevalent HLA-B27 subtypes with response to treatment.
References:
1. Bowness P. Annu Rev Immunol 2015;33:29-48
2. Lin H. Rheumatol Int 2017;37:1267-1280
3. Poddubnyy D, et al. ARD 2020;79:1624-1625
To cite this abstract in AMA style:
Poddubnyy D, Pournara E, Schulz B, Sadhu S, Deodhar A, Baraliakos X, Marzo-Ortega H. Efficacy of Secukinumab and HLA-B27 Subtypes: Results from a Phase IIIb Randomised Controlled Trial in Axial SpA [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-secukinumab-and-hla-b27-subtypes-results-from-a-phase-iiib-randomised-controlled-trial-in-axial-spa/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-secukinumab-and-hla-b27-subtypes-results-from-a-phase-iiib-randomised-controlled-trial-in-axial-spa/