Session Information
Date: Sunday, November 8, 2020
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Interstitial lung disease (ILD) is a fatal complication associated with connective tissue diseases (CTDs), often resulting in substantial morbidity and mortality. Despite numerous advances in immunosuppressive agents, there remains limited data on effective treatment for this challenging entity. This study aims to evaluate the efficacy of rituximab (RTX) in patients with CTD-ILD and determine factors correlated with outcomes at 6, 12, 24 and 36 months post-RTX.
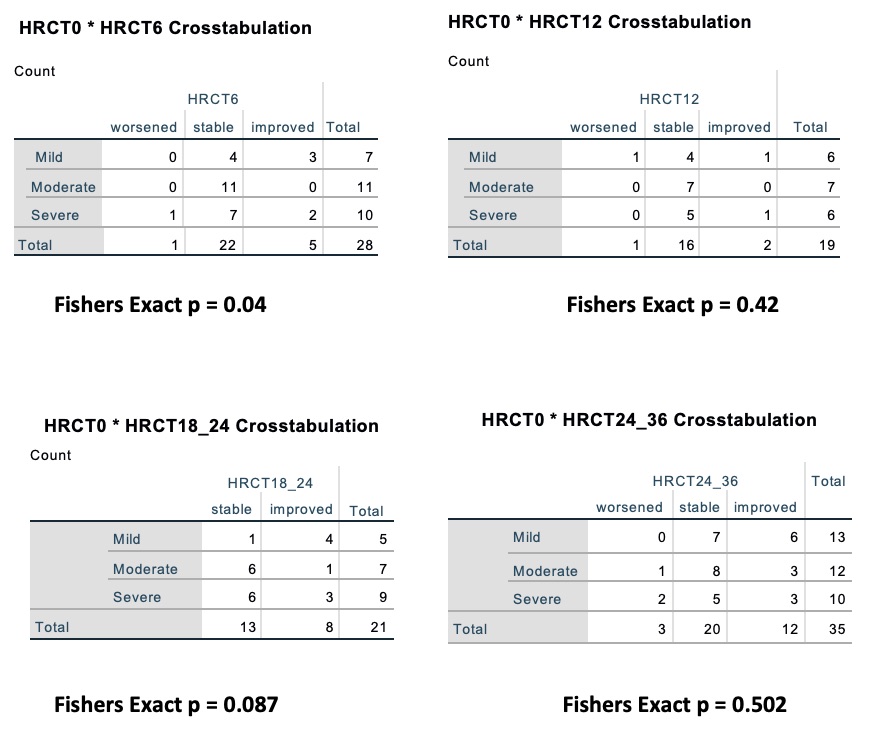
Methods: We analyzed data for 47 patients with CTD-ILD, all of whom met ACR classification criteria for a specific CTD. ILD was confirmed by high-resolution CT chest (HRCT) and pulmonary function tests with forced vital capacity (FVC) and diffusion capacity of lung for carbon monoxide (DLCO). We compared HRCT chest findings, FVC and DLCO at time of diagnosis and at 6, 12, 24 and 36 months post-RTX. At diagnosis HRCT chest findings classified into 3 groups (mild, moderate and severe). At 6, 12, 24 and 36 months after RTX, using the same semi-quantitative scoring system, HRCT chest findings were ranked as worsening, stable or improving.
Multiple patient characteristics (Table 1) were tested for their correlation with each outcome. For some variables, nonparametric statistical methods were used due to a small number of non-missing variables. The Spearman rank correlation and Wilcoxon signed rank test were used to determine changes in FVC and DLCO at 6, 12, 24 and 36 months after RTX treatment.
Results:
- Majority of the patients were female and African American with median age of 60.
- HRCT Chest findings after RTX showed either improvement or stability at 6, 12, 24 months, 3 cases showed worsening at 36 months, two of them missed one or two doses of Rituxan, and the 3rd cases was a longstanding ILD secondary to Scleroderma (Table 2).
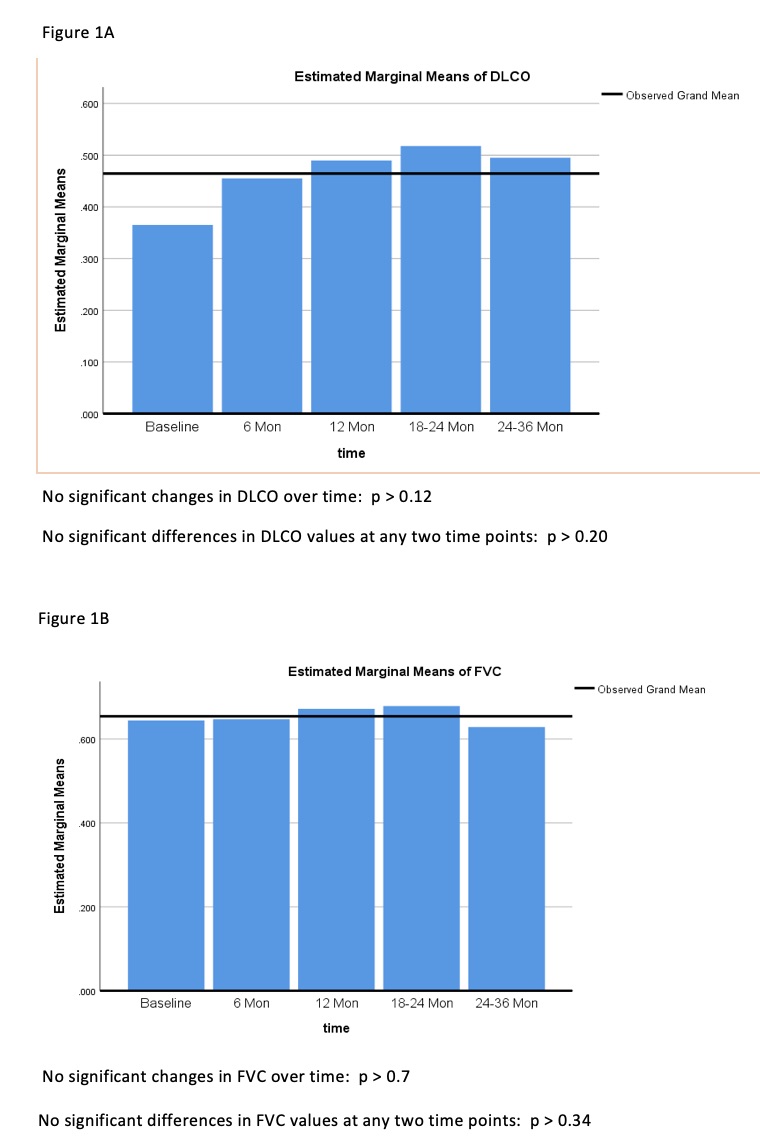
- There was no statistical significance at p-value for changes in FVC and DLCO, however observed changes have shown improvement as evidenced by observed grand mean. The largest changes were observed for FVC and DLCO at 1 year and DLCO at 2 years after treatment (Figure 1A, Figure 1B).
- CTD duration showed negative correlation with FVC change at 1 year post-RTX with estimated 1.1% decrease in FVC for every year increase in CTD. ILD duration showed negative correlation with DLCO change at 2 years post-RTX with estimated 3.9% decrease in DLCO for every year increase in ILD.
Conclusion: Based on this single center retrospective study, RTX appears to be an effective therapy for CTD-ILD. Our observations also suggest using RTX earlier in the disease course of CTD-ILD may have a greater long-term impact. RTX may help to fill an unmet therapeutic need for CTD-ILD but larger randomized clinical trials are needed
 Summary Statistics On Patient Characteristics and Outcomes (N=47)
Summary Statistics On Patient Characteristics and Outcomes (N=47)
To cite this abstract in AMA style:
Qurie A, Maruvada S, Umer S, Mclarty J, Hayat S. Efficacy of Rituximab for Connective Tissue Disease (CTD) Associated Interstitial Lung Disease (ILD) : A Single Center Study of 47 Patients- Extension Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-rituximab-for-connective-tissue-disease-ctd-associated-interstitial-lung-disease-ild-a-single-center-study-of-47-patients-extension-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-rituximab-for-connective-tissue-disease-ctd-associated-interstitial-lung-disease-ild-a-single-center-study-of-47-patients-extension-study/