Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Mirogabalin is a novel, preferentially selective a2d-1 ligand intended for treatment of pain associated with fibromyalgia and neuropathic pain. Mirogabalin efficacy and safety was evaluated in a randomized, double-blind, placebo-controlled and active comparator (pregabalin)-controlled, adaptive proof-of-concept phase 2 study in patients with diabetic peripheral neuropathic pain (DPNP) (Diabetes Care 2014;37:3253–61). Mirogabalin administered once or twice daily (5-30 mg/d) showed early and sustained reductions in average daily pain score (ADPS) relative to placebo (P<0.05 for 15, 20, and 30 mg/d) after 5 wk of treatment. Pain reduction with pregabalin 300 mg/d was not significantly different from placebo at end of 5 wks’ treatment. The most common adverse events (AEs) with mirogabalin were dizziness (9.4%), somnolence (6.1%), and headache (6.1%). Here we report key secondary outcomes of mirogabalin on patient-reported pain and sleep interference.
Methods: Adults (≥18 years) with type 1 or 2 diabetes, HbA1c ≤10% at screening, and DPNP for ≥6 months were eligible. Subjects (N=452) were randomly assigned (2:1:1:1:1:1:1 ratio) to receive placebo, dose-ranging mirogabalin (5, 10, 15, 20, or 30 mg/d), or pregabalin (300 mg/d) for 5 wk. Secondary efficacy end points included modified brief pain inventory (BPI) score, and average daily sleep interference score (ADSIS).
Results: At wk 5, statistically significant reduction in ADSIS was observed in the mirogabalin 15, 20, and 30 mg/d groups, compared with the placebo group (Fig 1). Baseline and mean changes from baseline in ADSIS and ADPS values at wk 5 (Fig 2) were strongly correlated. Four of the 6 BPI subscales showed significant differences in change from baseline to end point in at least 2 of the mirogabalin groups (15 and 30 mg/d), compared with placebo (P<0.05). Overall, a low incidence of treatment-related AEs was reported for mirogabalin.
Conclusion: These results support the efficacy of mirogabalin for improving patient-reported pain and sleep interference in patients with DPNP. Results support a direct impact on pain at the 3 highest tested doses of mirogabalin in this DPNP population. Current data did not support an indirect effect of mirogabalin on pain via an improvement of sleep, in contrast to what has been reported for pregabalin. This may be owing to duration of the current study and variability of results observed at tested doses of pregabalin across the larger and longer duration DPNP pregabalin studies. Investigation is underway in large phase 3 studies to clarify the relationship in pain reduction and sleep improvement. Figure 1. Mean change in ADSIS from baseline to end of treatment. 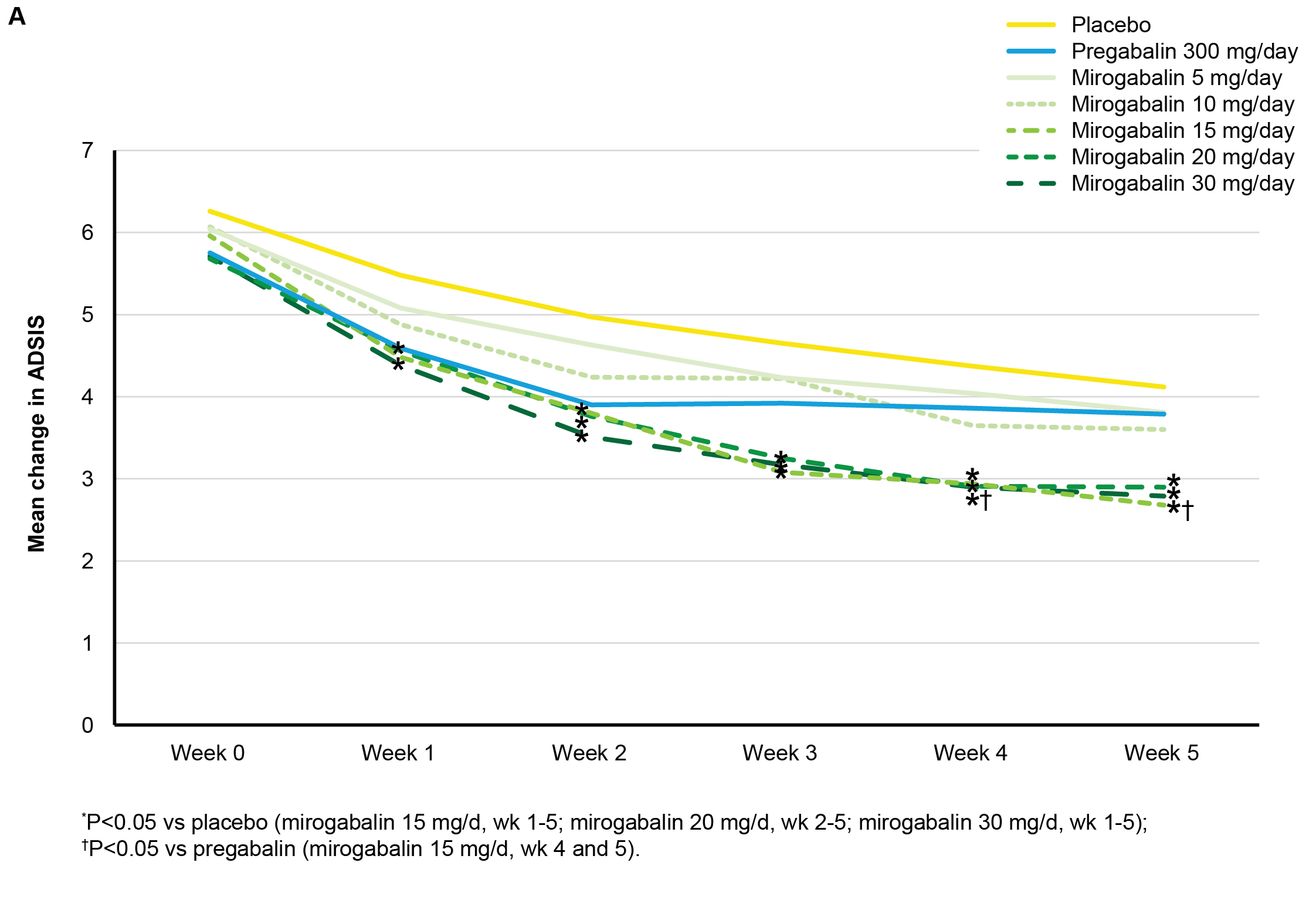 Figure 2. Correlation plot of weekly ADPS change from baseline and ADSIS change from baseline at wk 5 (LOCF).
Figure 2. Correlation plot of weekly ADPS change from baseline and ADSIS change from baseline at wk 5 (LOCF). 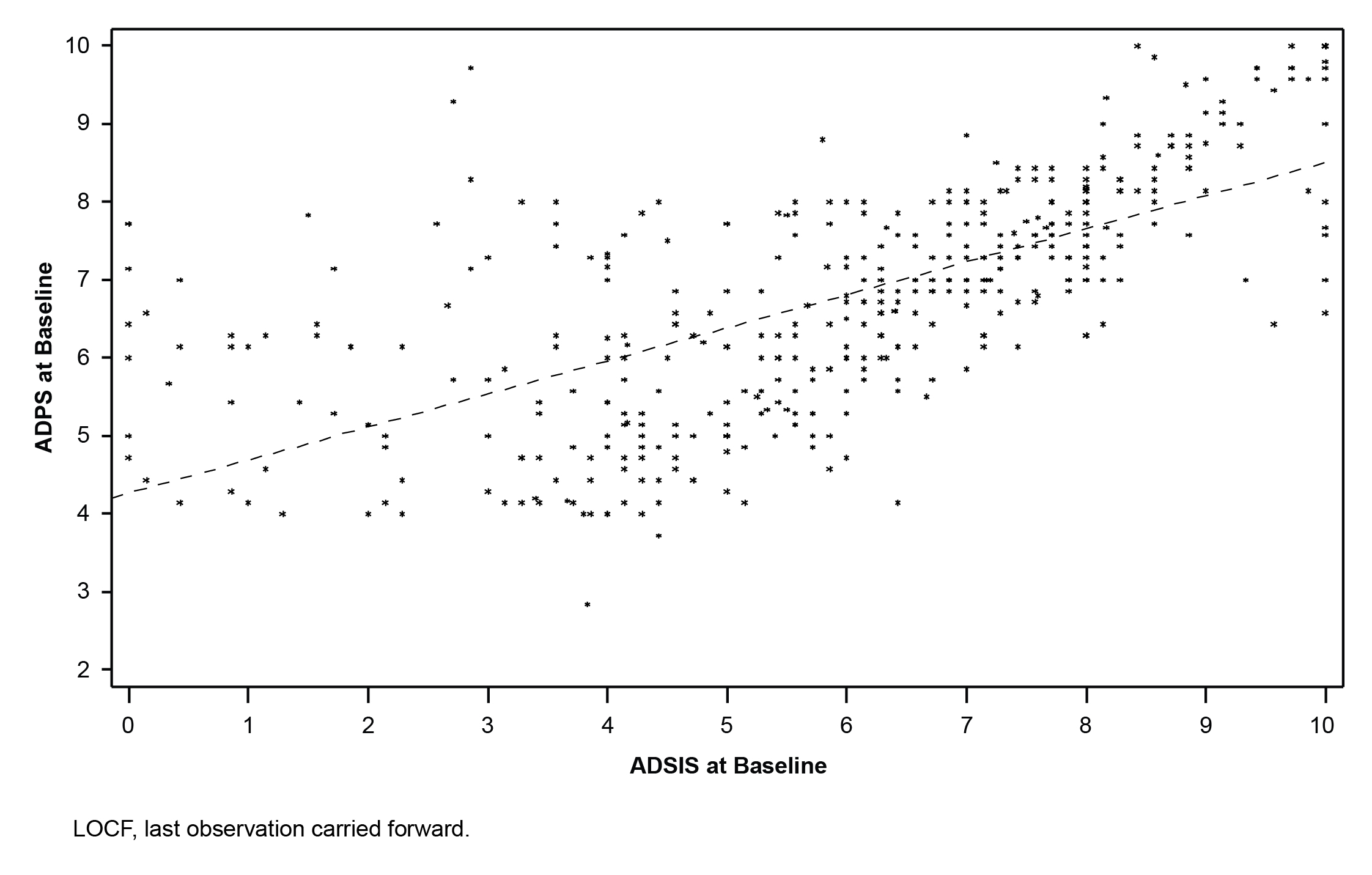
To cite this abstract in AMA style:
Merante D, Rosenstock J, Sharma U, Feins K, Hsu C, Vinik A. Efficacy of Mirogabalin on Patient-Reported Pain and Sleep Interference in Patients with Diabetic Neuropathic Pain: Secondary Outcomes of a Phase 2 Proof-of-Concept Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-mirogabalin-on-patient-reported-pain-and-sleep-interference-in-patients-with-diabetic-neuropathic-pain-secondary-outcomes-of-a-phase-2-proof-of-concept-study/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-mirogabalin-on-patient-reported-pain-and-sleep-interference-in-patients-with-diabetic-neuropathic-pain-secondary-outcomes-of-a-phase-2-proof-of-concept-study/
