Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Ixekizumab (IXE), a selective interleukin-17A antagonist, is approved for the treatment of active PsA, moderate-to-severe psoriasis (PsO), and radiographic/non-radiographic axial SpA in adults. The efficacy of IXE was compared to adalimumab (ADA) in patients (pts) with PsA and concomitant PsO in the SPIRIT-H2H (NCT03151551) study. Here we report results at weeks (wks) 24 and 52 from a subgroup analysis based on baseline PsO severity.
Methods: SPIRIT-H2H was a 52-wk, multicenter, randomized, open-label, rater-blinded, parallel-group study of biologic DMARD-naïve pts (N=566) with PsA and active PsO (≥3% body surface area involvement). Pts were randomized (stratified by concomitant use of conventional synthetic DMARDs and PsO severity) to IXE or ADA. Pts received on label dosing according to the severity of PsO. We report efficacy outcomes at wks 24 and 52 for the subgroup analysis of patients with/without moderate-to-severe PsO at baseline. The primary endpoint was the proportion of pts simultaneously achieving ≥50% improvement in ACR criteria (ACR50) and 100% improvement in PASI (PASI100) at wk 24. Additional post-hoc analysis was performed for other endpoints. Logistic regression models were performed with treatment, baseline PsO severity and treatment-by-baseline PsO severity interaction as independent variables. Missing data were imputed using non-responder imputation. Differences in the proportion of responders between IXE and ADA in each subgroup were assessed using Fisher’s exact test.
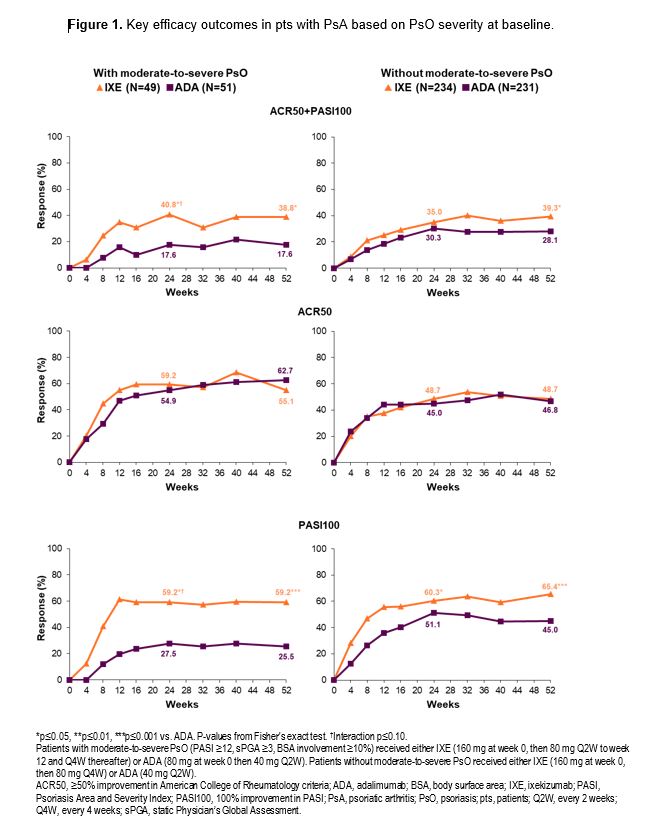
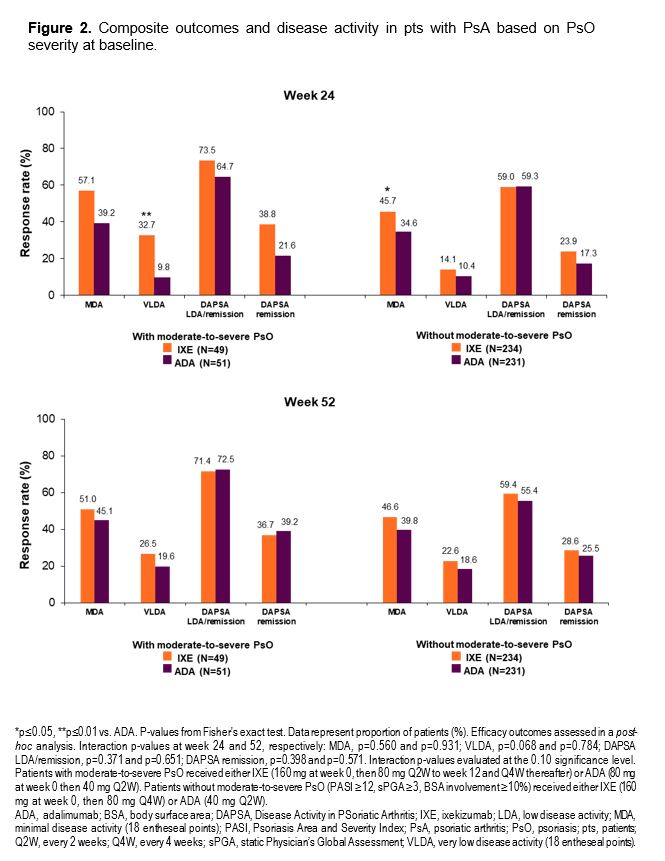
Results: At baseline, 49/283 IXE-treated pts and 51/282 ADA-treated pts had moderate-to-severe PsO. A greater proportion of IXE-treated pts achieved the combined endpoint of ACR50+PASI100, and PASI100 compared to ADA at wks 24 and 52, regardless of baseline PsO severity (Figure 1). Similar efficacy was observed on the joints for IXE and ADA across both pt subgroups (Table). Faster improvement was observed for IXE than for ADA with respect to minimal disease activity (MDA) and Disease Activity in Psoriatic Arthritis (DAPSA) remission regardless of PsO severity, and for very low disease activity (VLDA) in pts with moderate-to-severe PsO (Figure 2).
Conclusion: In pts with active PsA, a significantly higher proportion of IXE-treated pts achieved the combined ACR50+PASI100 endpoint, and PASI100 at wk 52 compared to ADA, regardless of baseline PsO severity. ACR50 response at wks 24 and 52 was not influenced by different IXE dosing. Faster improvements in MDA and DAPSA remission were observed with IXE than with ADA.these subgroup analyses were consistent with data from the overall SPIRIT-H2H population.
To cite this abstract in AMA style:
Kristensen L, Okada M, Tillett W, Liu-Leage S, El Baou C, Bradley A, Meszaros G, de Vlam K. Efficacy of Ixekizumab versus Adalimumab in Psoriatic Arthritis (PsA) Patients with and Without Moderate-to-severe Psoriasis: 52-week Results from a Multicentre, Randomised Open-label Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-ixekizumab-versus-adalimumab-in-psoriatic-arthritis-psa-patients-with-and-without-moderate-to-severe-psoriasis-52-week-results-from-a-multicentre-randomised-open-label-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-ixekizumab-versus-adalimumab-in-psoriatic-arthritis-psa-patients-with-and-without-moderate-to-severe-psoriasis-52-week-results-from-a-multicentre-randomised-open-label-study/