Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: In previous DISCOVER (D)1 and 2 analyses, the fully human IL-23p19-subunit inhibitor guselkumab (GUS) was associated with robust and sustained improvement in PsA signs/symptoms over 52 weeks (W) in subgroups of patients (pts) across several baseline (BL) demographic and disease characteristics.1 GUS efficacy explicitly in PsA pts with severe disease activity (DA) has not been assessed. Here, GUS efficacy through W100 in D2 bionaive PsA pts with severe DA based on a range of composite and pt-reported outcomes was evaluated.
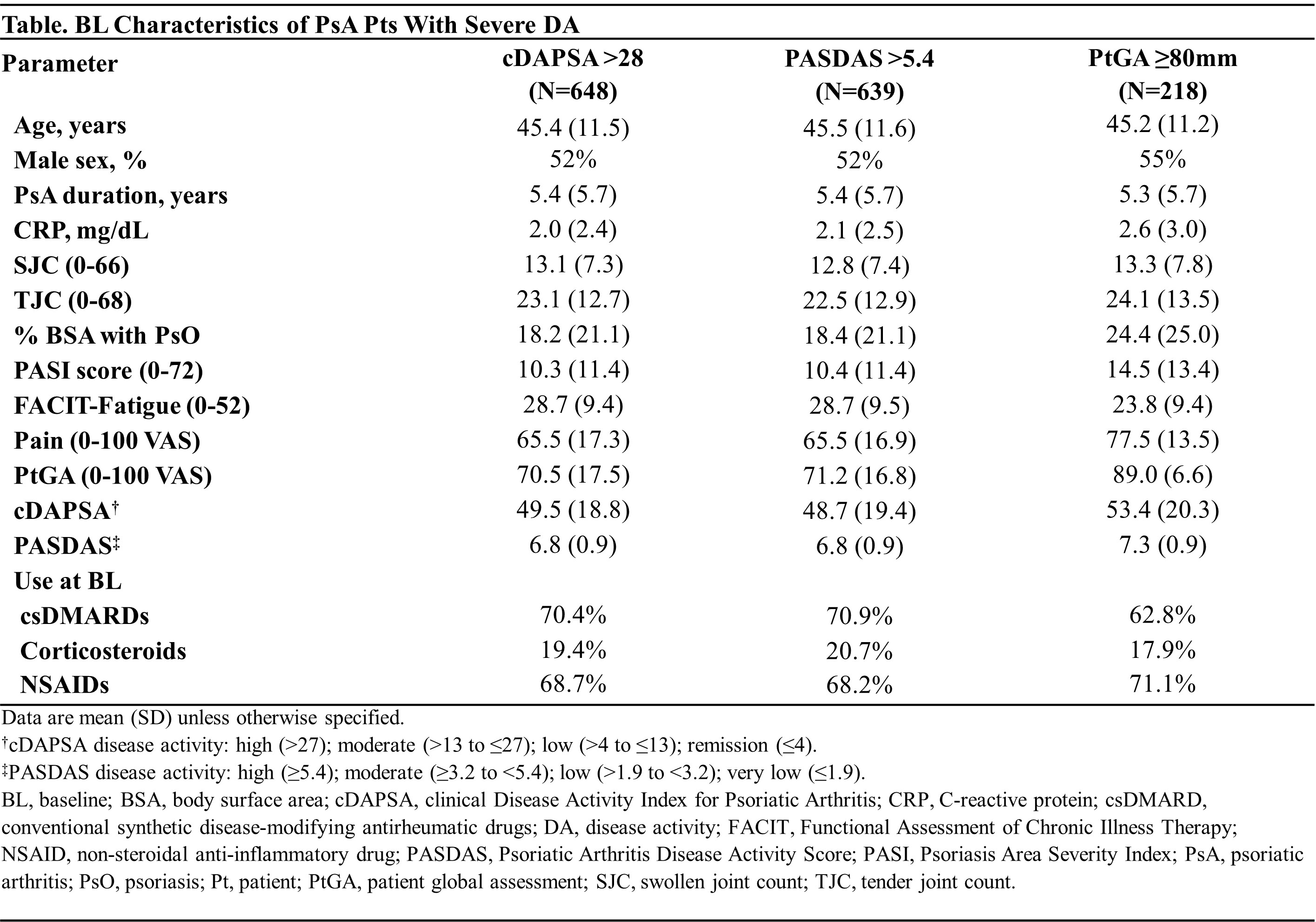
Methods: D2 enrolled bionaive adults with active PsA (≥5 swollen [SJC] and ≥5 tender [TJC] joints, CRP ≥0.6 mg/dL). Pts were randomized (1:1:1) to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, Q8W; or placebo (PBO)→GUS Q4W at W24. Severe DA was defined based on clinical DA Index for PsA (cDAPSA >28), PsA DA Score (PASDAS >5.4), and pt global assessment (PtGA ≥80 mm) criteria. cDAPSA was the sum of individual scores for SJC, TJC, PtGA, and pt assessment of pain; PASDAS included SJC, TJC, dactylitis and enthesitis scores, CRP, physician global assessment of disease activity, PtGA, and the physical component of the 36-Item Short Form Health Survey. Least square mean changes from BL for each outcome were estimated with mixed models for repeated measures, adjusted for treatment group, BL outcome levels, and study stratification factors (BL csDMARD use and high-sensitivity serum CRP value).
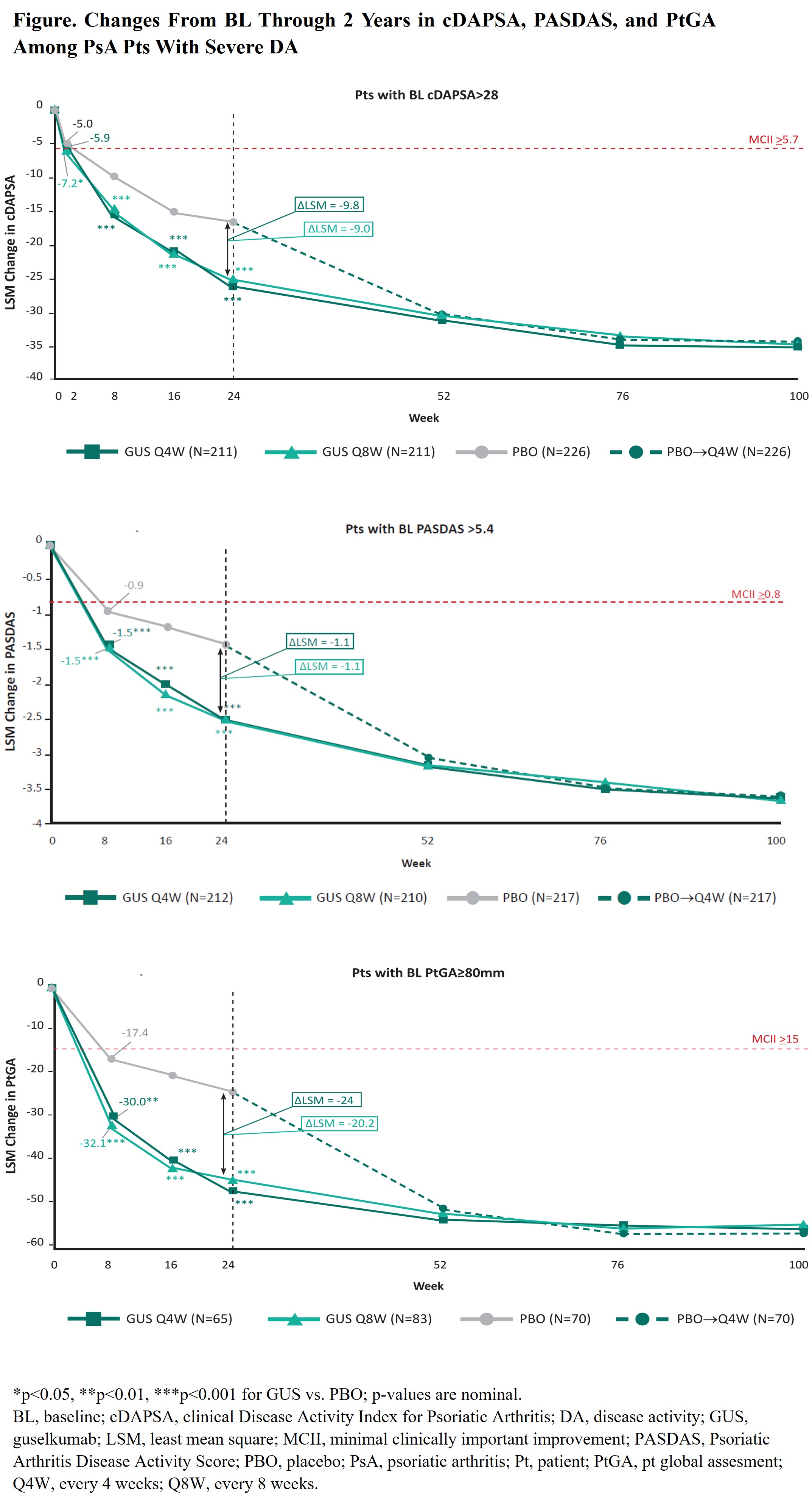
Results: Of 739 pts, 648 (88%), 639 (86%), and 218 (29%) met the cDAPSA (mean=49.5), PASDAS (mean=6.8), and PtGA (mean=89.0) criteria for severe DA, respectively. BL characteristics were generally consistent across cohorts (Table). Treatment groups were generally balanced across severe DA cohorts, except GUS Q4W pts were more likely to be male and have dactylitis compared with PBO pts (data not shown). In multivariate analyses, irrespective of severe DA cohort, GUS-treated pts experienced significantly greater improvements than PBO pts in all outcomes (largest effect seen in the severe PtGA cohort [Figure]). On average, improvements with GUS were clinically meaningful as of the first assessment timepoint, specifically W2 for cDAPSA (GUS Q4W: -5.9; GUS Q8W: -7.2; PBO: -5.0) and W8 for PASDAS (GUS Q4W: -1.5; GUS Q8W: -1.5; PBO: -0.9) and PtGA (GUS Q4W: -30.0; GUS Q8W: -32.1; PBO: -17.4; Figure). Differences vs PBO were enhanced through W24 across severe DA cohorts, at which time further improvements with GUS Q4W/Q8W were observed, resulting in differences vs PBO of -9.8/-9.0, -1.1/-1.1, -24.0/-20.2 in cDAPSA, PASDAS, and PtGA, respectively. Further improvements in all outcomes (ΔcDAPSA ̴ -36; ΔPASDAS ̴ -3.6; ΔPtGA ̴ ‑56) were observed through 2 years of GUS treatment, representing ̴73% improvement in joint DA, ̴53% improvement in PsA activity across domains, and ̴63% improvement in pt-reported overall DA.
Conclusion: In bionaive PsA pts with severe DA based on joint-focused and multi-domain outcomes, GUS led to rapid (joint: W2; overall DA: W8) clinically meaningful improvements in DA; these improvements were further enhanced by W24 and sustained through 2 years. Findings support the use of GUS to treat PsA pts with severe DA.
1Ritchlin CT. RMD Open. 2022;8:e002195
To cite this abstract in AMA style:
Ritchlin C, Lubrano E, Chimenti M, Leibowitz E, Sharaf M, Adelakun O, Rampakakis E, Nantel F, Lavie F, Deodhar A. Efficacy of Guselkumab in Bionaive Psoriatic Arthritis Patients with Severe Disease Activity: Post-hoc Analysis of a Phase 3, Randomized, Double-Blind, Placebo-Controlled Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-guselkumab-in-bionaive-psoriatic-arthritis-patients-with-severe-disease-activity-post-hoc-analysis-of-a-phase-3-randomized-double-blind-placebo-controlled-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-guselkumab-in-bionaive-psoriatic-arthritis-patients-with-severe-disease-activity-post-hoc-analysis-of-a-phase-3-randomized-double-blind-placebo-controlled-study/