Session Information
Date: Monday, November 9, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment II: Emerging Therapies (2023–2027)
Session Type: Abstract Session
Session Time: 11:00AM-11:50AM
Background/Purpose: Guselkumab (GUS), an interleukin-23 inhibitor, improved axial symptoms of active psoriatic arthritis (PsA) through week 24 in a pooled analysis from two phase 3 trials (DISCOVER-1 and DISCOVER-2).1 We now report the efficacy of GUS through 1 year in PsA patients (pts) with imaging-confirmed sacroiliitis in DISCOVER-1&2.
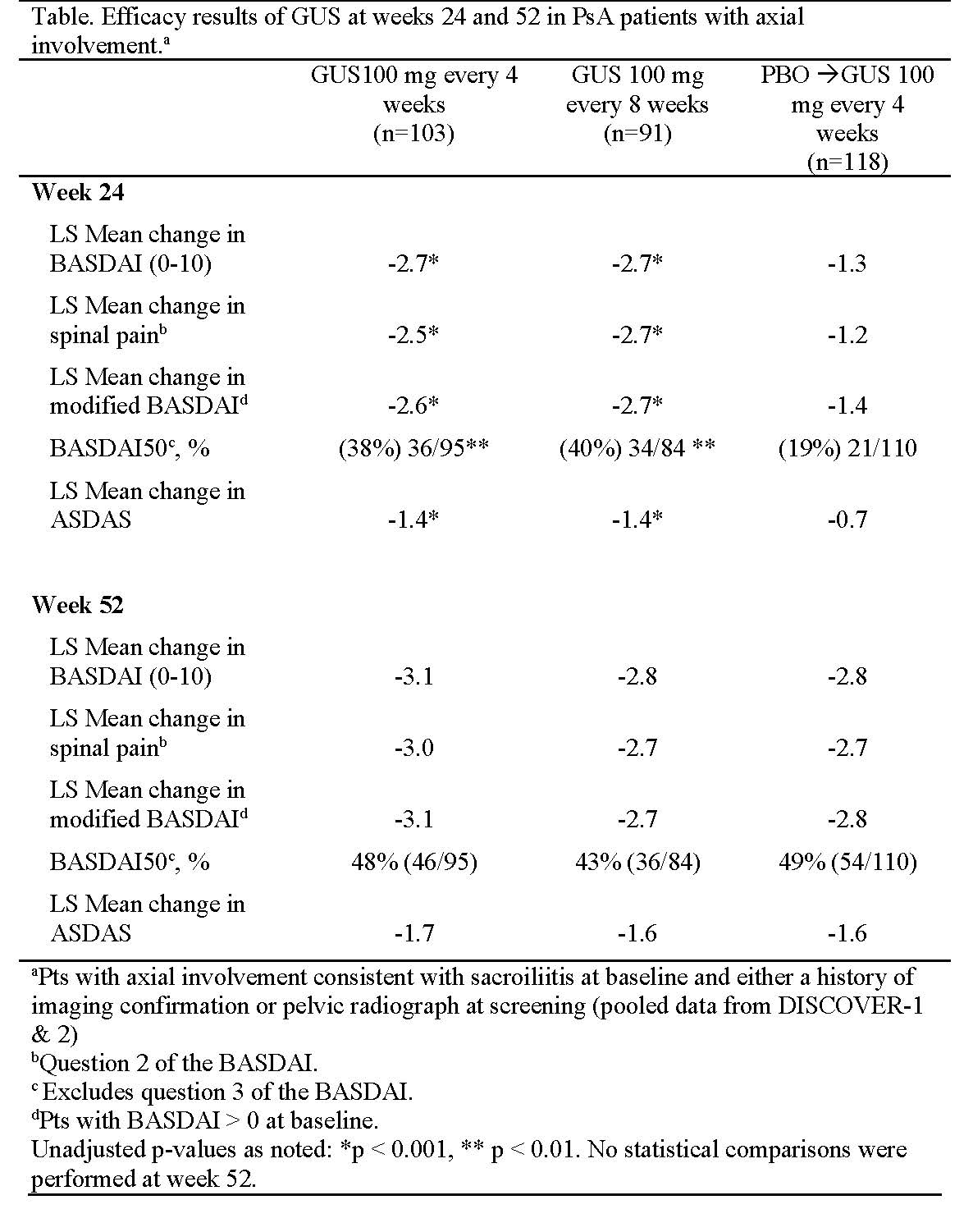
Methods: In DISCOVER-1, 381 pts with active PsA (≥ 3 swollen joints, ≥ 3 tender joints; C-reactive protein ≥ 0.3mg/dL despite standard therapies) and in DISCOVER-2, 739 pts with active PsA (≥ 5 swollen joints, ≥ 5 tender joints, CRP ≥ 0.6mg/dL despite standard therapies) were randomized 1:1:1 to GUS 100mg q4w, GUS 100mg q8w (wk0, wk4, then q8w), or placebo (PBO). PBO pts crossed over to GUS 100mg q4w at wk24. Only pts with sacroiliitis at baseline who had either documented imaging confirmation of sacroiliitis in the past or pelvic radiograph confirmation of sacroiliitis at screening (based on investigators’ judgment) were included in this analysis (pooled data from DISCOVER 1&2). Efficacy was assessed by BASDAI score, BASDAI50, modified BASDAI (mBASDAI; excludes Q#3), spinal pain (BASDAI Q#2), ASDAS(-CRP) score, and ASDAS responses of inactive disease (< 1.3), major improvement (decrease ≥2.0), and clinically important improvement (decrease ≥1.1) through week 52. For response endpoints, pts who met treatment failure rules or had missing data were counted as nonresponders through week 24; pts with missing data were counted as nonresponders from weeks 24-52. For changes in scores, a change of 0 was assigned for treatment failures through week 24, and pts who discontinued or had missing data were set to 0 for weeks 24-52. HLA-B27 was assayed in a subset of 190 of these pts.
Results: 312 pts across both studies presented with imaging confirmed sacroiliitis (PBO, n = 118; GUS q8w, n = 91; GUS q4w, n = 103). At baseline, mean BASDAI and ASDAS scores ranged from 6.5 – 6.6 and 3.9 – 4.0, respectively; 57/190 (30%) pts were HLA-B27+, and 133/190 (70%) were HLA-B27-. Improvements in axial symptoms of PsA were greater in the GUS q4w and q8w groups vs PBO through week 24. The LS mean changes from baseline in BASDAI, spinal pain, mBASDAI, and ASDAS were maintained from week 24 to week 52 in the GUS groups (Table); improvements from baseline to week 52 in the PBO pts who crossed over to GUS were similar to those in the GUS groups. Similar trends were observed for the proportions of pts achieving BASDAI50 (Table) and ASDAS responses of inactive disease, major improvement, and clinically important improvement (Figure) at week 52. Efficacy at week 52 trended similarly between HLA-B27+ and HLA-B27- pts.
Conclusion: Improvements in axial symptoms were maintained through week 52 in GUS-treated pts with active PsA who had imaging-confirmed sacroiliitis.
Reference:
- Helliwell P, Gladman D, Poddubnyy D, et al. Efficacy of Guselkumab, a Monoclonal Antibody that Specifically Binds to the p19 Subunit of IL-23, on Endpoints Related to Axial Involvement in Patients with Active PsA with Imaging-Confirmed Sacroiliitis: Week-24 Results from Two Phase 3, Randomized, Double-blind, Placebo-controlled Studies. EULAR 2020.
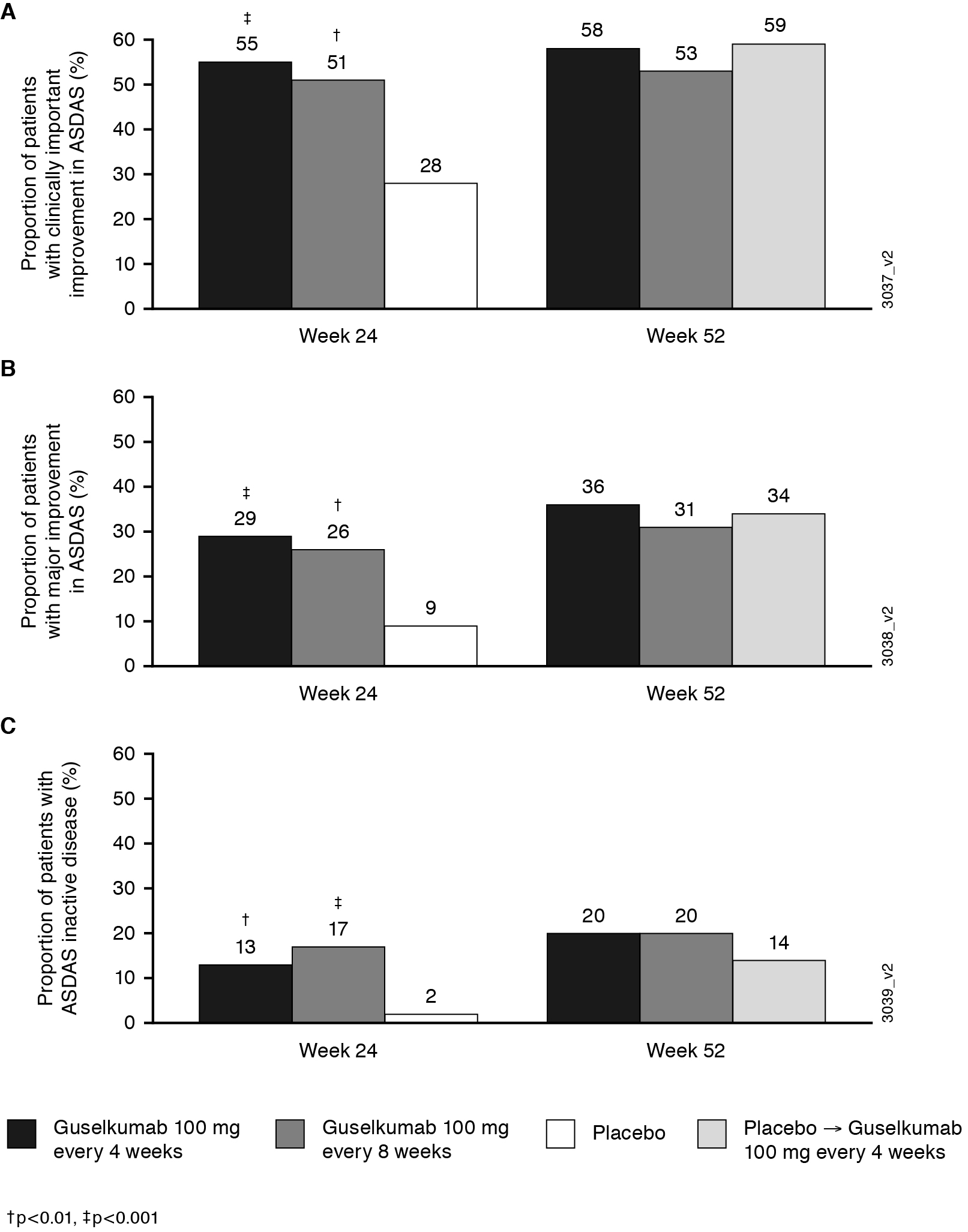 Figure. Proportion of patients with ASDAS clinically important improvement, major improvement, and inactive disease
Figure. Proportion of patients with ASDAS clinically important improvement, major improvement, and inactive disease
To cite this abstract in AMA style:
Mease P, Helliwell P, Gladman D, Poddubnyy D, Baraliakos X, Chakravarty S, Kollmeier A, Hsia E, Xu X, Sheng S, Agarwal P, Zhou B, Shawi M, Karyekar C, Sweet K, Deodhar A, van der Heijde D. Efficacy of Guselkumab, a Monoclonal Antibody That Specifically Binds to the p19 Subunit of IL-23, on Axial-Related Endpoints in Patients with Active PsA with Imaging-Confirmed Sacroiliitis: Week-52 Results from Two Phase 3, Randomized, Double-blind, Placebo-controlled Studies [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-guselkumab-a-monoclonal-antibody-that-specifically-binds-to-the-p19-subunit-of-il-23-on-axial-related-endpoints-in-patients-with-active-psa-with-imaging-confirmed-sacroiliitis-week-52-r/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-guselkumab-a-monoclonal-antibody-that-specifically-binds-to-the-p19-subunit-of-il-23-on-axial-related-endpoints-in-patients-with-active-psa-with-imaging-confirmed-sacroiliitis-week-52-r/