Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Efficacy of golimumab (GLM) for nr-axSpA was demonstrated in a randomized, double-blind (DB), placebo (PBO)-controlled, phase 3 study (GO-AHEAD; NCT01453725).1 In a subgroup analysis, we now investigate the effects of GLM based on presence or absence of objective inflammation (sacroiliitis on MRI and/or C-reactive protein [CRP] > upper limit of normal [ULN]) at baseline.
Methods: Patients with nr-axSpA (ASAS criteria, centrally read SI joint X-rays/MRIs, disease duration ≤5 years, chronic back pain ≥3 years, high disease activity, and inadequate response or intolerance to NSAIDs) were randomized (with ASAS-defined MRI sacroiliitis by single central reader [yes, SI+; no, SI–] and CRP level [≤ULN or >ULN] as stratification factors) to GLM 50 mg SC or PBO Q4W for 16 weeks. The primary endpoint was ASAS20 response at week 16. Estimated between-group differences in response at week 16 on ASAS20, ASAS40, BASDAI50, and ASAS partial remission (PR) were compared for four patient subgroups (MRI SI+ & CRP >ULN; MRI SI– & CRP >ULN; MRI SI+ & CRP ≤ULN; MRI SI– & CRP ≤ULN) by Miettinen-Nurminen methods; no multiplicity control was used.
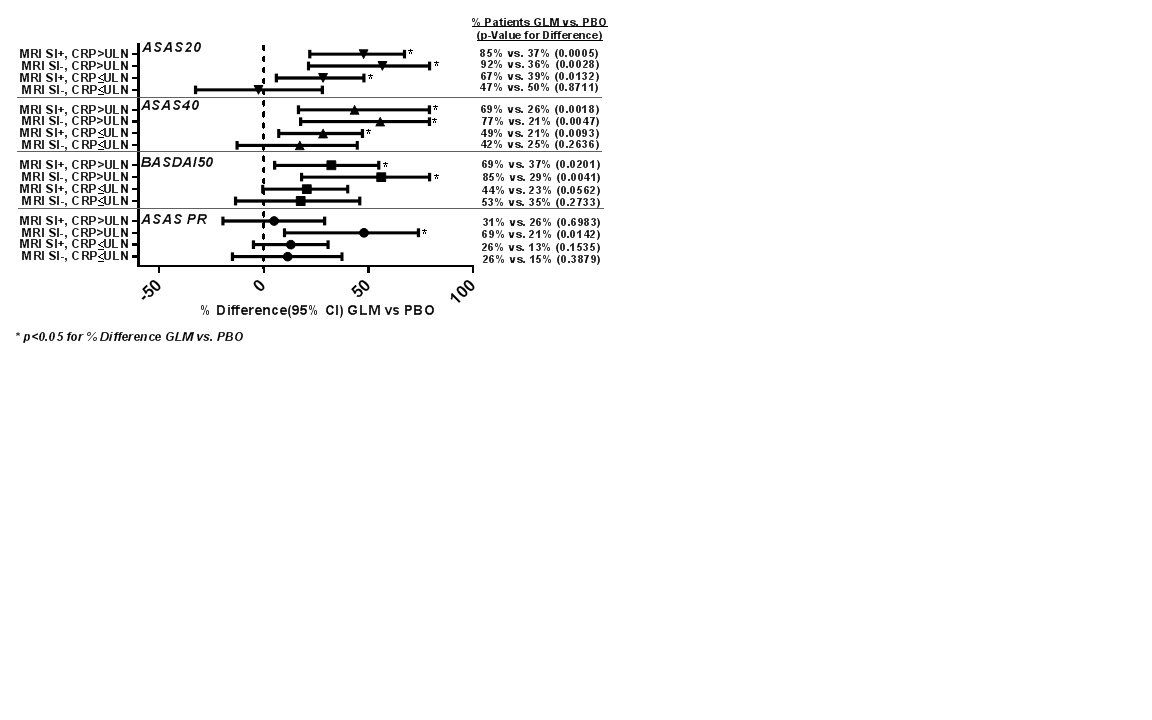
Results: In total, 197 patients were treated (GLM=97; PBO=100). Treatment-group differences in ASAS20, ASAS40, BASDAI50, and ASAS PR response were greater in patients with baseline objective inflammation (Figure). Results should be interpreted with caution, given the small subgroups and absence of multiplicity control.
Conclusion: In the GO-AHEAD trial, responses to GLM (vs PBO) were greater in patients with objective inflammation (particularly with CRP >ULN) at baseline. Reference 1. Sieper J, et al. Arthritis Rheum. 2015;67(10),2702–2712. Figure: Subgroup Analysis of Response by MRI SI Status and CRP Level at Baseline
SUBMISSION INFORMATION Conflict of Interest Disclosures: JS: Consulting: AbbVie, Eli Lilly, Janssen Biologics, Merck, Novartis, Pfizer, Roche, UCB DVH: Consultant: AbbVie, Amgen, AstraZeneca, Augurex, BMS, Boehringer Ingelheim, Celgene, Centocor, Chugai, Covagen, Daiichi, Eli-Lilly, Galapagos, GSK, Janssen Biologics, Merck, Novartis, Novo-Nordisk, Otsuka, Pfizer, Roche, Sanofi-Aventis, UCB, Vertex; employment: Imaging Rheumatology BV MD: Grant/research support: AbbVie, Lilly, Novartis, Pfizer, Roche, Sanofi, UCB WPM: Grant/research support: AbbVie, Janssen, Pfizer; consultant: AbbVie, Amgen, UCB, Pfizer, Merck, Janssen, Eli Lilly, Celgene, Synarc, Boehringer GB, SPC, GP, AT, SH: Employment, shareholders: Merck & Co., Inc., Kenilworth, NJ, USA JB: Grant/research support: Abbvie (Abbott), Amgen, Biogen, Boehringer, BMS, Celgene, Celltrion, Centocor, Chugai, EBEWE Pharma, Epirus, Hospira, Janssen, Medac, MSD (Schering-Plough), Mundipharma, Novartis, Pfizer (Wyeth), Roche, Sanofi-Aventis and UCB; Consulting: Abbvie (Abbott), Amgen, Biogen, Boehringer, BMS, Celgene, Celltrion, Centocor, Chugai, EBEWE Pharma, Epirus, Hospira, Janssen, Medac, MSD (Schering-Plough), Mundipharma, Novartis, Pfizer (Wyeth), Roche, Sanofi-Aventis and UCB.
To cite this abstract in AMA style:
Sieper J, van der Heijde D, Maksymowych W, Braun J, Bergman G, Curtis SP, Tzontcheva A, Philip G, Huyck S, Dougados M. Efficacy of Golimumab for Nonradiographic Axial Spondyloarthritis (nr-axSpA): Subgroup Analysis By Baseline MRI and C-Reactive Protein Status [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-golimumab-for-nonradiographic-axial-spondyloarthritis-nr-axspa-subgroup-analysis-by-baseline-mri-and-c-reactive-protein-status/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-golimumab-for-nonradiographic-axial-spondyloarthritis-nr-axspa-subgroup-analysis-by-baseline-mri-and-c-reactive-protein-status/