Session Information
Date: Monday, November 9, 2020
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: There is conflicting evidence for methotrexate (MTX) efficacy in giant cell arteritis (GCA).1,2 Subanalysis of data from the 52-week, double-blind, randomized controlled GiACTA trial in patients with GCA who received tocilizumab (TCZ) or placebo (PBO) with prednisone tapering was performed to assess efficacy and safety in patients who received adjunctive MTX.
Methods: In GiACTA, patients were randomly assigned to receive subcutaneous TCZ every week or every other week plus 26-week prednisone tapering or PBO every week plus 26-week or 52-week prednisone tapering for 52 weeks.3 The two TCZ groups and the two PBO groups were pooled for this analysis. Stable doses of MTX could be initiated at screening, continued in the double-blind period, and reduced/discontinued at the investigator’s discretion. Efficacy was defined as sustained remission (absence of GCA flare and C-reactive protein < 1 mg/dL from weeks 12 to 52 and adherence to the prednisone taper).3 ACR GCA classification criteria were fulfilled by 78% of enrolled patients. The study was conducted in accordance with the principles of the Declaration of Helsinki and received IRB approval.
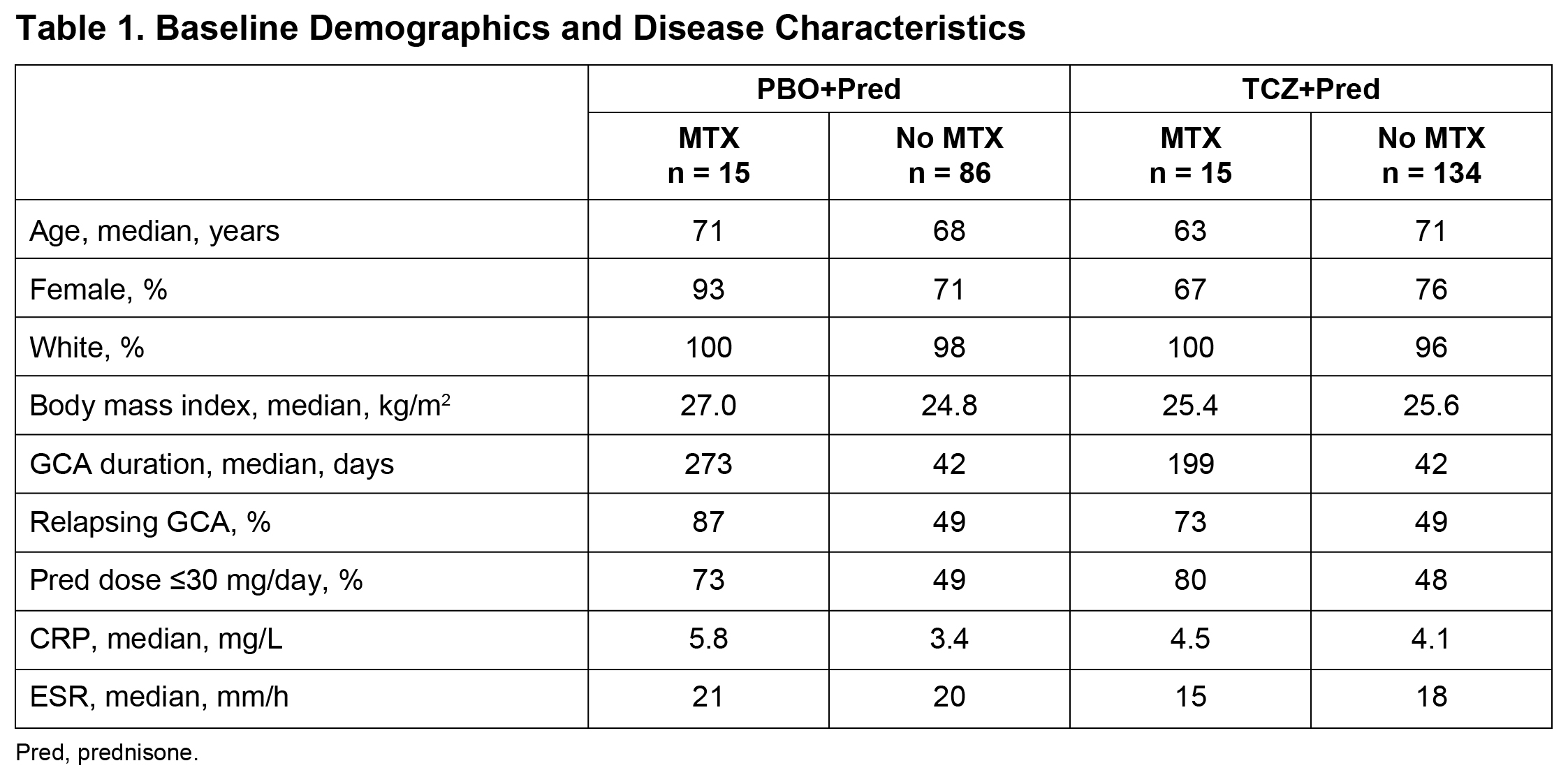
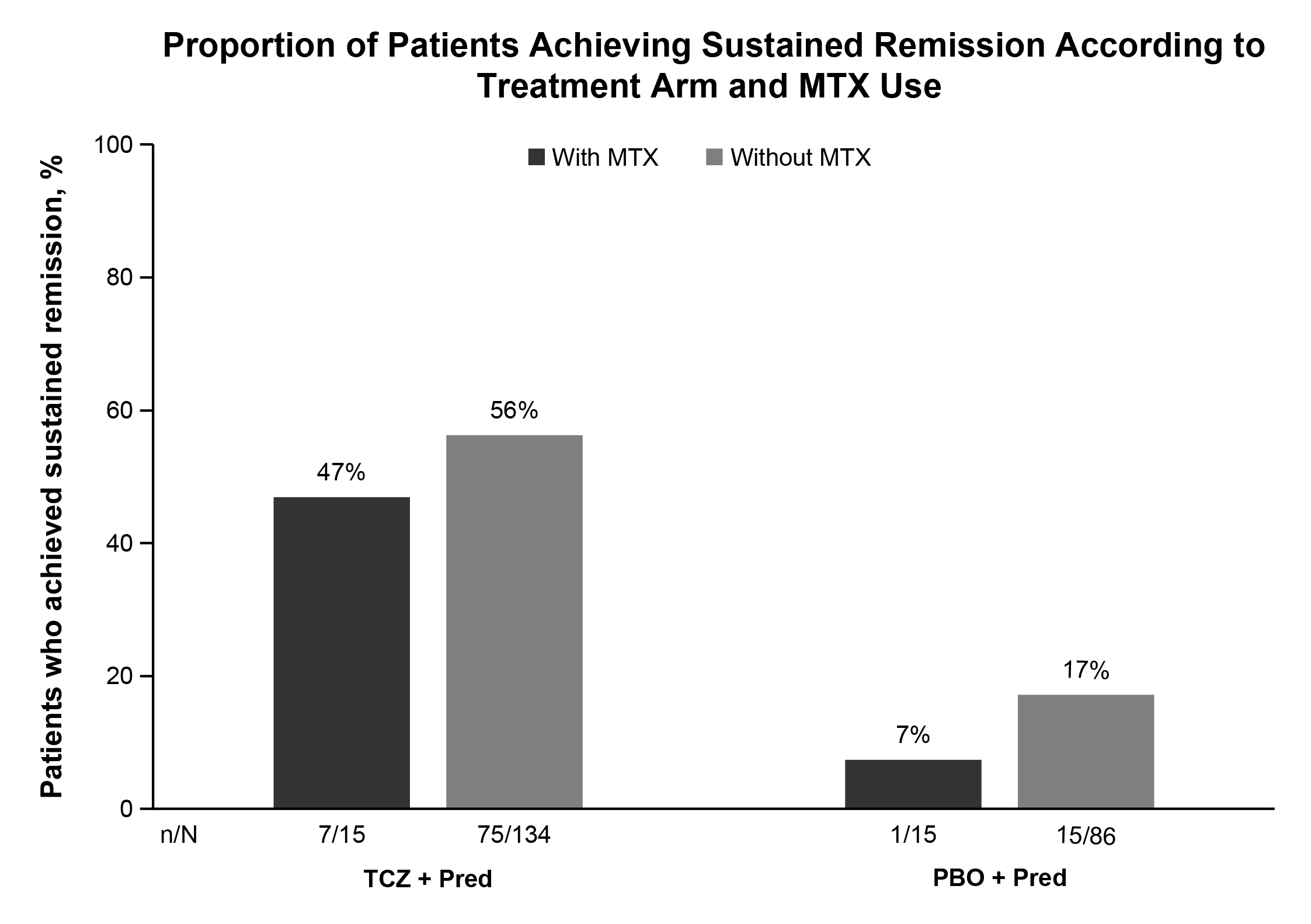
Results: Overall, 30 of 250 (12%) treated patients received adjunctive MTX for a median duration of 52.1 weeks: 15 of 149 (10%) TCZ-treated patients received MTX for a median of 52.1 weeks and 15 of 101 (15%) PBO-treated patients received MTX for a median of 52.0 weeks. Baseline characteristics (Table 1) were balanced, except for longer disease duration, higher proportion of patients with relapsing GCA, and lower baseline prednisone doses among patients who received MTX. The median cumulative glucocorticoid dose over 52 weeks was similar between patients who received MTX and those who did not in the PBO groups (2730 mg and 3694 mg, respectively) and the TCZ groups (1385 mg and 1862 mg, respectively). Sustained remission was achieved by 7 of 15 (47%) patients treated with MTX and 75 of 134 (56%) patients without MTX in the TCZ groups and by 1 of 15 (7%) patients treated MTX and 15 of 86 (17%) patients without MTX in the PBO groups (Figure 1). In the primary analysis,3 82 of 149 (55%) patients in the TCZ groups and 16 of 101 (16%) patients in the PBO groups achieved sustained remission. The mean annualized relapse rate at 52 weeks was not different between MTX-treated and MTX-untreated patients for the TCZ (0.71 with MTX vs 0.47 without MTX; p = 0.3305) or PBO (1.77 vs 1.48; p = 0.6019) groups. Adverse event rates per 100 patient-years were numerically higher in MTX-treated than MTX-untreated patients: 1207 and 862, respectively, in the TCZ groups and 1270 and 957, respectively, in the PBO groups.
Conclusion: Preliminary data from a small subgroup of patients suggest that adjunctive MTX does not increase the likelihood of sustained remission, reduce disease relapse rate, or improve steroid sparing in patients with GCA. Response rates in TCZ-treated patients appear to be independent of treatment with MTX. Results from this post hoc analysis should be confirmed in larger studies.
References: 1. Hoffman GS et al. Arthritis Rheum 2002;46:1309-18; 2. Mahr AD et al. Arthritis Rheum 2007;56:2789-97; 3. Stone JH et al. N Engl J Med 2017;377:317-28.
To cite this abstract in AMA style:
Stone J, Han J, Mohan S. Efficacy of Adjunctive Methotrexate in Patients with Giant Cell Arteritis Treated with Tocilizumab Plus Prednisone Tapering: Subanalysis of a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-adjunctive-methotrexate-in-patients-with-giant-cell-arteritis-treated-with-tocilizumab-plus-prednisone-tapering-subanalysis-of-a-phase-3-trial/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-adjunctive-methotrexate-in-patients-with-giant-cell-arteritis-treated-with-tocilizumab-plus-prednisone-tapering-subanalysis-of-a-phase-3-trial/