Session Information
Date: Sunday, November 10, 2019
Title: Measures Of Healthcare Quality Poster I: Testing, Screening, & Treating
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Biotechnological drugs are a fundamental resource for the treatment (Tx) of rheumatic patients (Pts). Biosimilar drugs are intended to be as effective as the originator product but with a lower cost to healthcare systems.
In our center we promoted a switch from originator infliximab (IFXor) to biosimilar infliximab (CT-P13). We analyzed efficacy, safety, immunogenicity and cost savings of switching.
Methods: Eligible Pts were those older than 18 years old with the diagnosis of rheumatoid arthritis (RA), spondylarthritis (SpA) and psoriatic arthritis (PsA) on Tx with IFXor for at least 6 months and with stable disease activity (DA). In December 2016 all eligible Pts were proposed to switch to CT-P13. At the day of the last Tx with IFXor, informed consent, data and blood samples were collected. On the next Tx day, CT-P13 was administered after standard evaluation of efficacy and safety. Efficacy was measured considering change from baseline in DAS in 28 joints for RA and PsA and in Ankylosing Spondylitis Disease Activity Score (ASDAS) for SpA. Disease worsening was considered when an increase of 1.2 from baseline in DAS28 or an increase of 1.1 in ASDAS occurred. Serum Infliximab levels (sIFX) were dichotomized as low (< 3 µg/mL) and high ( >6 µg/mL). Anti-drug antibody (ADA) levels were dichotomized into detectable ( >10 ng/ml) or non-detectable (< 10 ng/ml). CT-P13 Tx withdrawal and drug persistence were used as effectiveness outcomes. A cost analysis was done based on the purchasing prices of the 2 drugs at our center.
Results: During a period of 1 year switch to CT-P13 was performed in 60 Pts for non-medical reasons.
We had a total of 36 Pts with SpA, 16 with RA and 8 with PsA. 65% were females with a median age of 53 years, median disease duration previous to switch of 17 years and median time on IFXor Tx of 8 years.
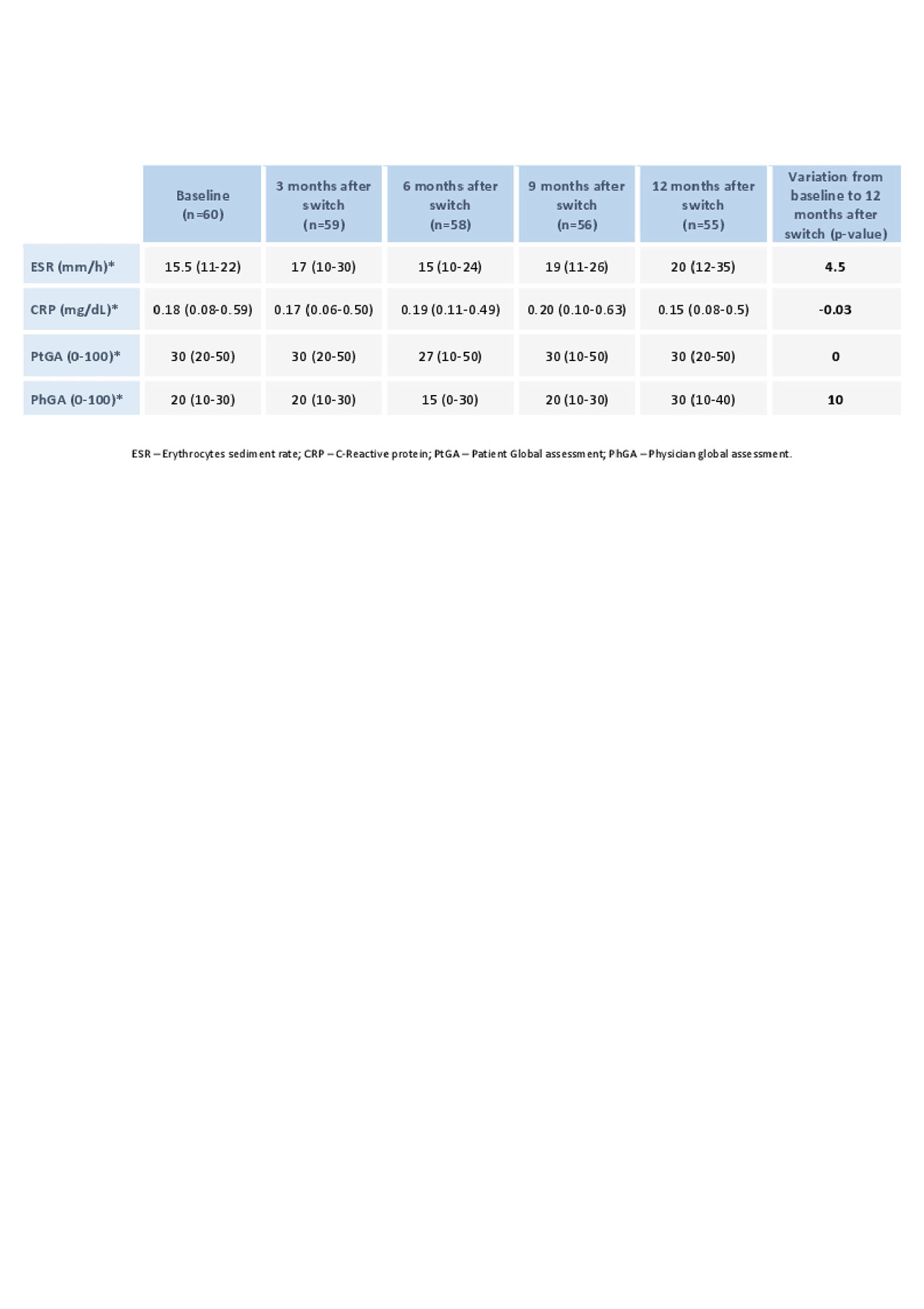
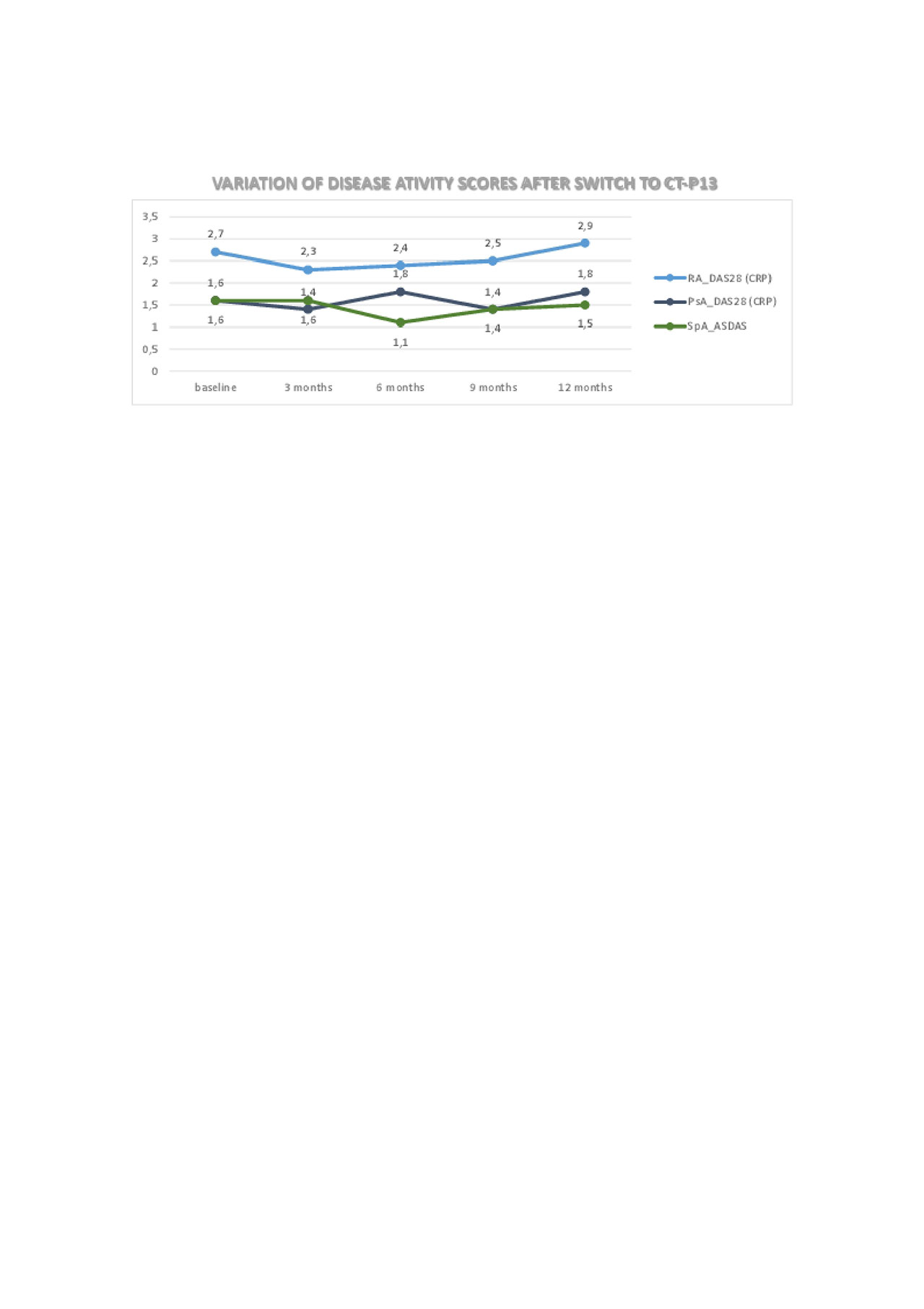
DA was stable over the observation period and similar to the values observed with IFXor. Median follow-up time was 15 months during which 5 Pts stopped CT-P13. Three Pts had disease worsening, 1 Pt had a minor adverse event (lip edema) and 1 Pt moved to another country. One patient returned to IFXor and the other 3 switched to another drug.
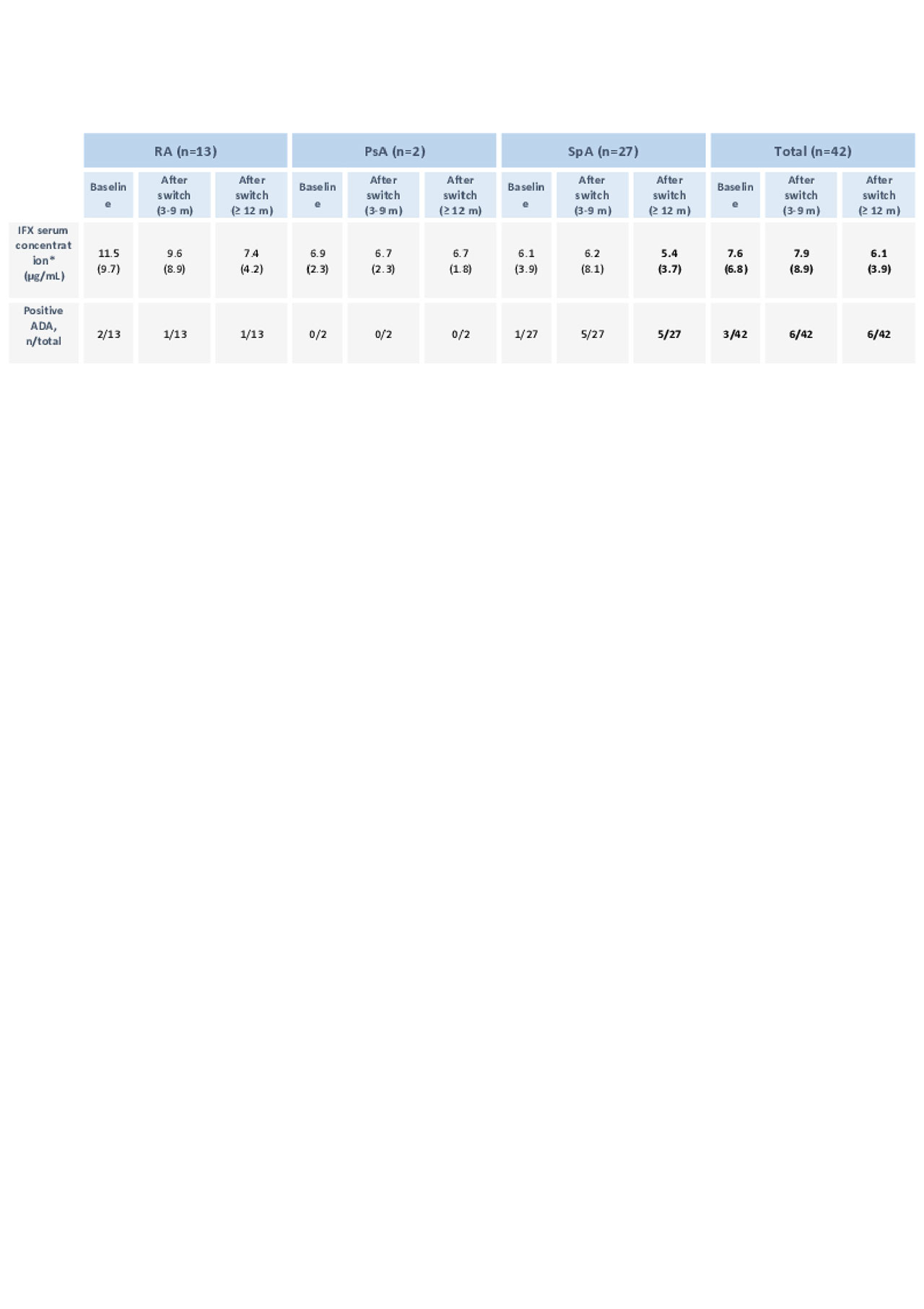
42 switchers had blood samples collected before and after switch. A total of 27 Pts had unaltered sIFX levels and ADA status during follow up.
At baseline, 5 Pts had low sIFX, with no detectable ADAs, that reverted after switch. These Pts had no variation in DA before and after switch.
3 Pts had detectable ADA at baseline with low sIFX levels. After switch, ADAs became negative in 2 of those Pts, with normalization of sIFX. The other Pt kept detectable ADA levels after switch and had a minor elevation of DAS28, based on patient global assessment.
ADAs became positive in 5 Pts after switch. Of these, sIFX changed from high to low accompanied by an elevation of ASDAS in 3 Pts. The other 2 Pts maintained ADA levels detectable with sIFX >3 µg/mL during all the evaluation period.
Apart from the Pts that developed ADAs, no other Pt changed from high to low sIFX.
The switch to CT-P13 represented a 26.4 % reduction of costs in the use of IFX Tx in these Pts.
Conclusion: The switch in routine care of a group of RA, SpA and PsA patients from IFXor to CT-P13 did not affect efficacy, safety or immunogenicity and reduced costs in 26.4%.
To cite this abstract in AMA style:
Valido A, Silva-Dinis J, Saavedra M, Iria I, Gonçalves J, Cruz J, Bernardo N, Eurico Fonseca J. Efficacy, Immunogenicity and Cost Analysis of a Systematic Switch from Originator Infliximab to Biossimilar CT-P13 of All Patients with Inflamatory Arthritis from a Single Center [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/efficacy-immunogenicity-and-cost-analysis-of-a-systematic-switch-from-originator-infliximab-to-biossimilar-ct-p13-of-all-patients-with-inflamatory-arthritis-from-a-single-center/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-immunogenicity-and-cost-analysis-of-a-systematic-switch-from-originator-infliximab-to-biossimilar-ct-p13-of-all-patients-with-inflamatory-arthritis-from-a-single-center/