Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: TYK2 mediates signaling by key cytokines involved in the pathogenesis of immune-mediated inflammatory diseases such as psoriatic arthritis (PsA) and psoriasis (PsO). TAK-279 is a highly selective, oral, allosteric TYK2 inhibitor shown to be clinically effective with an acceptable safety profile in a phase 2b trial in patients with moderate-to-severe PsO (Armstrong et al. AAD 2023).The current study evaluated efficacy and safety of TAK-279 in patients with active PsA treated over 12 weeks.
Methods: This phase 2b randomized, multicenter, double-blind, placebo (PBO)-controlled, dose-ranging study (NCT05153148) was conducted at 45 sites in North America and Europe. Eligible patients were aged ≥ 18 years, with PsA symptoms for ≥ 6 months prior to screening, met CASPAR criteria, and had ≥ 3 tender and ≥ 3 swollen joint counts (TJC/SJC) at enrollment despite prior NSAID, DMARD or biologic treatment. Patients were randomized 1:1:1:1 to receive oral TAK-279 5 mg, 15 mg, 30 mg, or PBO, once daily for 12 weeks. Primary endpoint: ACR 20 response at Week 12. Secondary endpoints at Week 12 included: ACR 50, ACR 70, PASI 75 responses (in patients with ≥ 3% BSA), change from baseline in TJC/SJC, physician global assessment (PhGA) of PsA, and safety.
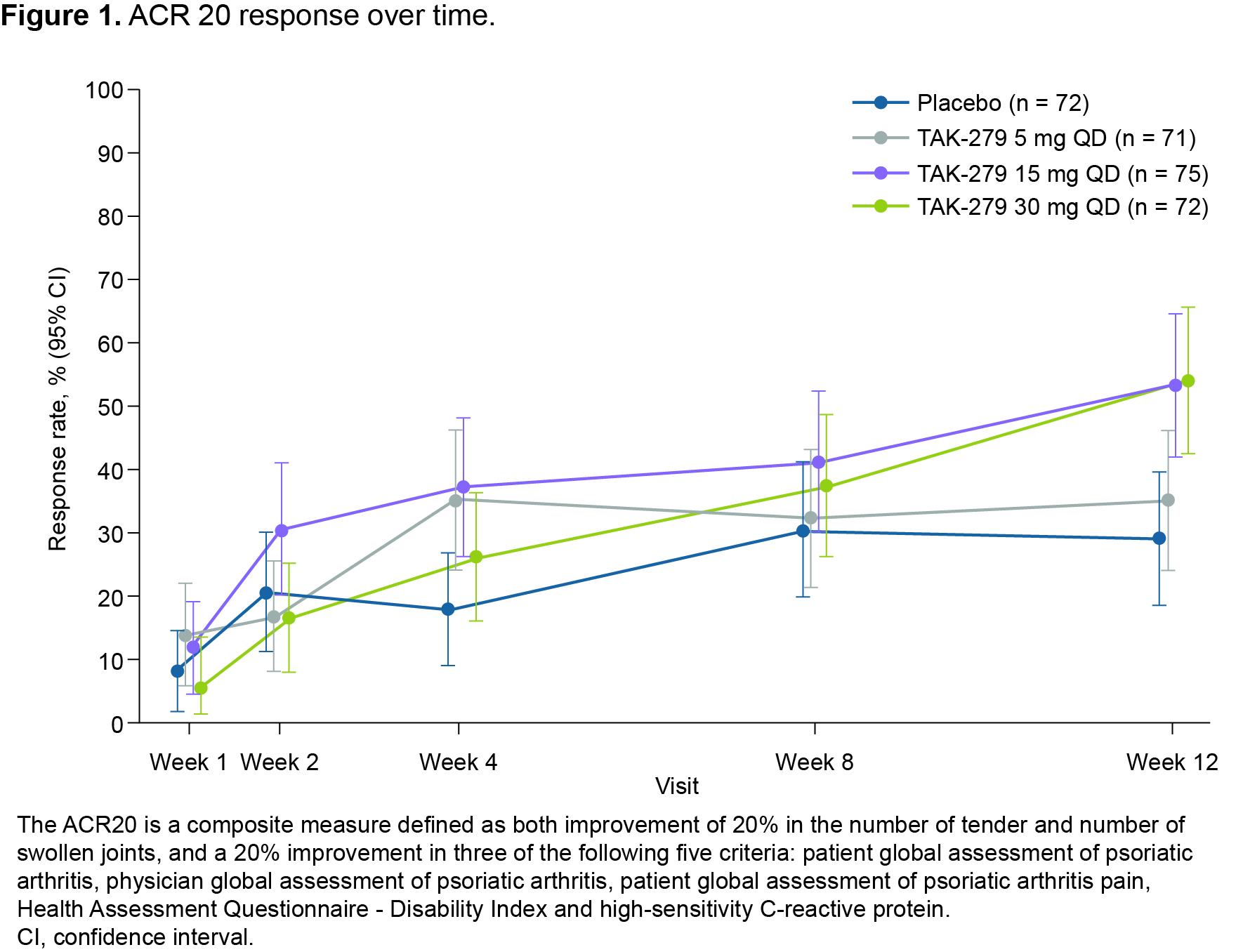
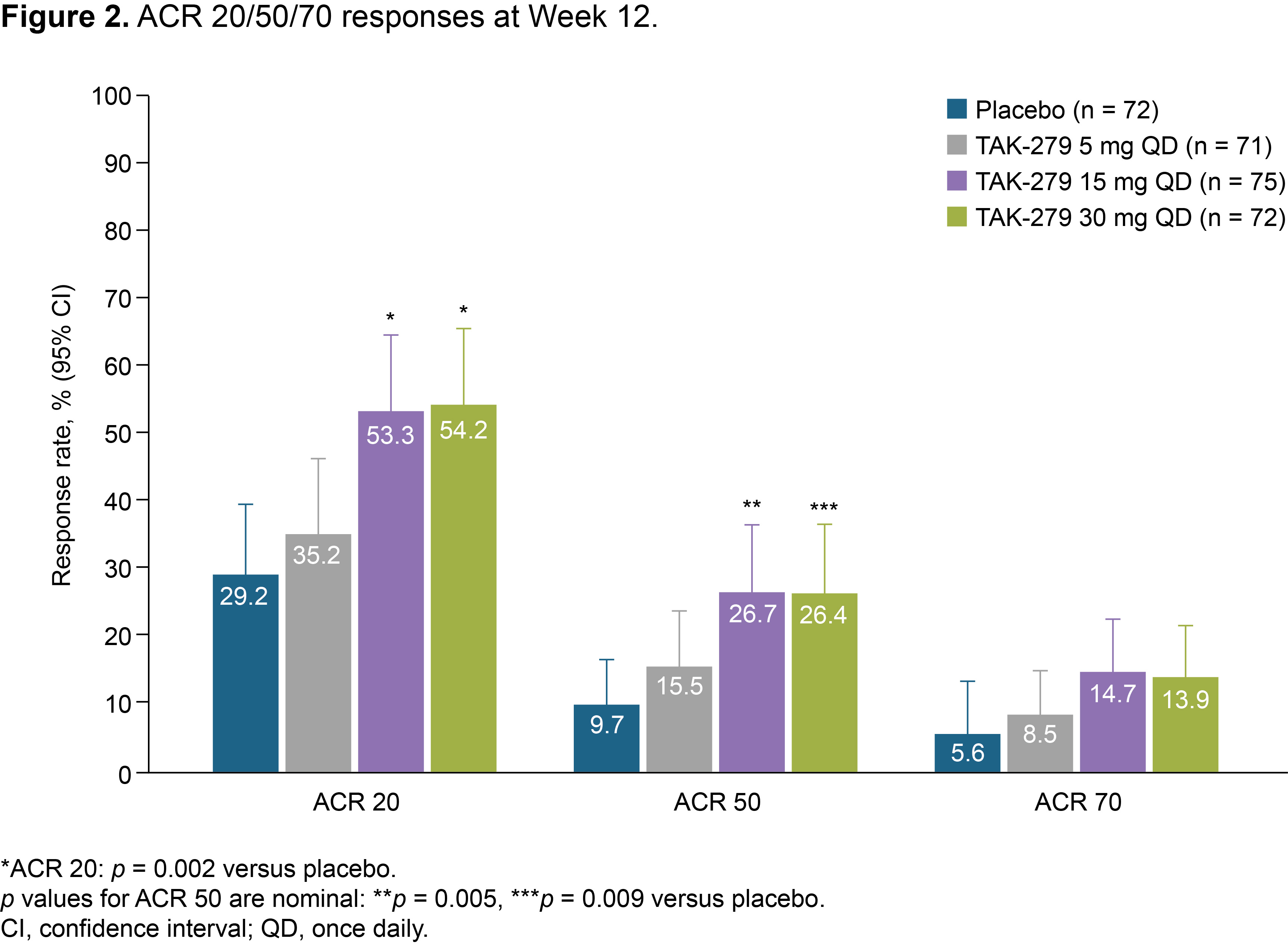
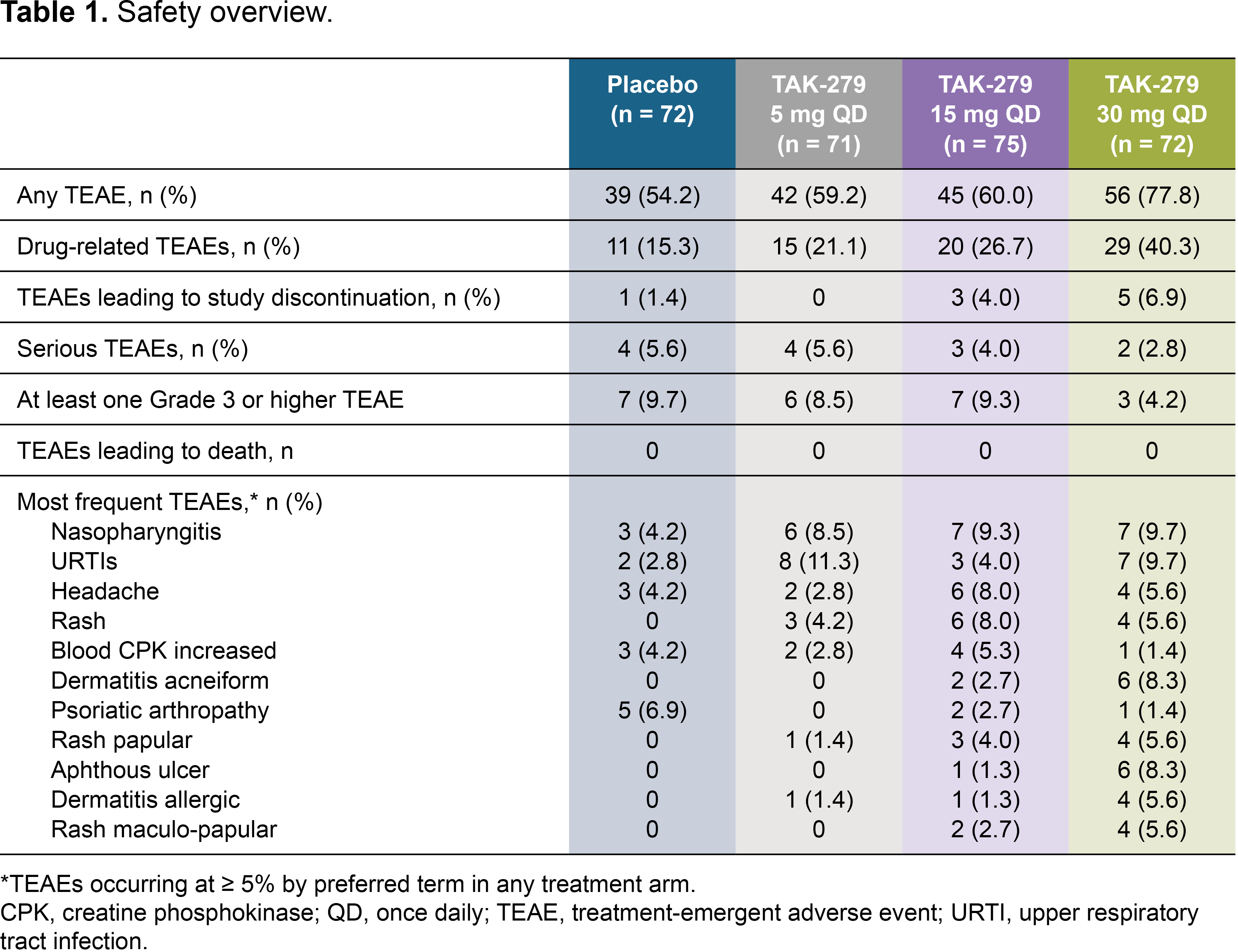
Results: In total, 290 patients were randomized and treated; 245 completed 12 weeks’ treatment. Baseline characteristics were generally comparable across groups (except for a lower mean TJC in the 30 mg group); 58.6% had BSA ≥ 3% (of which mean baseline PASI score was 6.2), and 32.1% had prior biologic use (20.7% TNFis). Mean baseline hsCRP was 7.0 mg/L; 45.9% had hsCRP ≥ 3 mg/L. The primary endpoint was met with a significantly greater proportion of patients achieving ACR 20 with TAK-279 15 mg and 30 mg vs PBO (53.3% and 54.2% vs 29.2%, both p = 0.002; Figure 1). ACR 50 response rates were also higher in the TAK-279 15 mg and 30 mg groups vs PBO (p = 0.005 and p = 0.009, respectively; Figure 2). PASI 75 response was highest in the 30 mg group vs other doses and PBO (45.7% [30 mg] and 28.3% [15 mg] vs 15.4% [PBO]; p = 0.002 and p = 0.101, respectively).A numerically higher proportion of patients treated with TAK-279 15 mg and 30 mg achieved ACR 70 than with PBO (Figure 2). Numerical reductions were observed in mean change from baseline in the TJC/SJC in all groups, with higher reductions with the 15 mg and 30 mg doses vs PBO and 5 mg TAK-279. Improvements from baseline were seen in PhGA of PsA in all TAK-279 groups vs PBO (5 mg [p = 0.016]; 15 mg [p = 0.004]; 30 mg [p = 0.003]). Safety outcomes are summarized in Table 1. Nasopharyngitis, upper respiratory tract infections, headache and rash were the most common treatment-emergent adverse events (TEAEs) in TAK-279-treated patients. No opportunistic infections, major adverse cardiovascular events or differences in mean laboratory parameters of interest were observed, compared with PBO. Serious and grade 3 or higher TEAEs occurred infrequently and at a similar rate in the TAK-279 and PBO groups.
Conclusion: TAK-279 was well tolerated and demonstrated superior dose-dependent efficacy to PBO over 12 weeks of treatment in patients with active PsA. Its safety profile was consistent with that observed in the phase 2b PsO study.
To cite this abstract in AMA style:
Kivitz A, Muensterman E, Kavanaugh A, van der Heijde D, Klimiuk P, Valenzuela G, Dokoupilova E, Poirier G, Srivastava B, Dasen S, Zhang X, Trivedi M, Weng H, Hong T, Pothula P, Baraliakos X. Efficacy and Safety Outcomes of TAK-279, a Selective Oral Tyrosine Kinase 2 (TYK2) Inhibitor, from a Randomized, Double-blind, Placebo-controlled Phase 2b Trial in Patients with Active Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-outcomes-of-tak-279-a-selective-oral-tyrosine-kinase-2-tyk2-inhibitor-from-a-randomized-double-blind-placebo-controlled-phase-2b-trial-in-patients-with-active-psoriatic-arthr/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-outcomes-of-tak-279-a-selective-oral-tyrosine-kinase-2-tyk2-inhibitor-from-a-randomized-double-blind-placebo-controlled-phase-2b-trial-in-patients-with-active-psoriatic-arthr/