Session Information
Date: Monday, November 9, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment II: Emerging Therapies (2023–2027)
Session Type: Abstract Session
Session Time: 11:00AM-11:50AM
Background/Purpose: Upadacitinib (UPA), an oral, reversible, JAK inhibitor approved to treat rheumatoid arthritis (RA), is under evaluation for psoriatic arthritis (PsA). We assess efficacy and safety of UPA vs placebo (PBO) and adalimumab (ADA) in patients (pts) with PsA and prior IR or intolerance to ≥1 non-biologic DMARD (non-bDMARD).
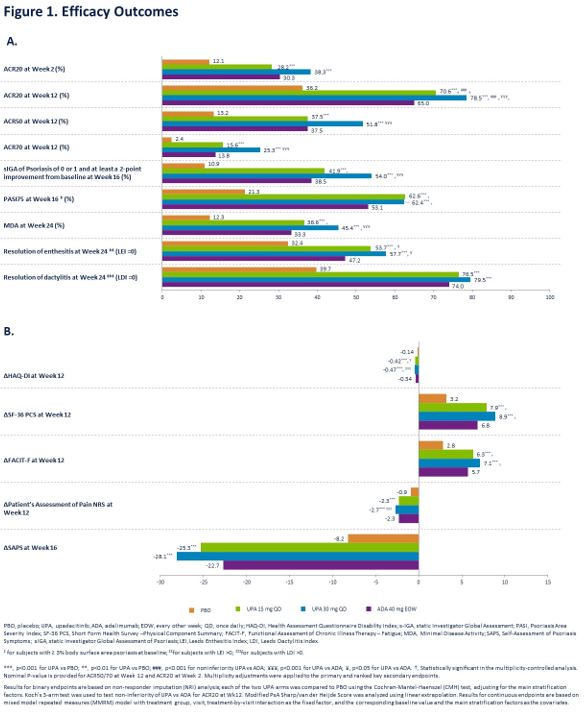
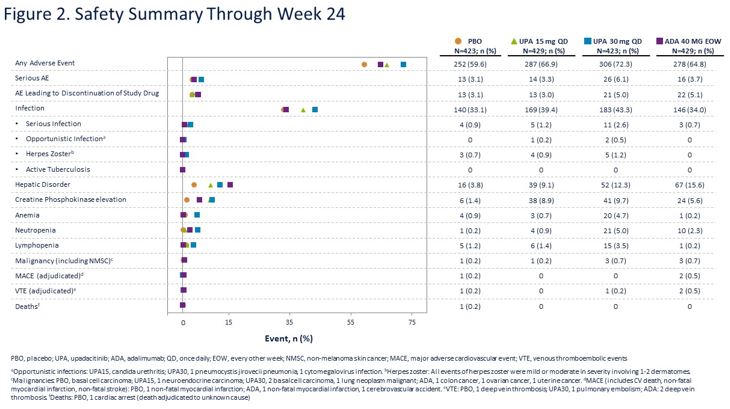
Methods: Pts with active PsA (≥3 swollen and ≥3 tender joints), active or historical psoriasis, and on ≤2 non-bDMARDs were randomized 1:1:1:1 to once daily UPA 15 mg (UPA15), UPA 30 mg (UPA30), ADA 40 mg every other week, or PBO. Primary endpoint was proportion of pts achieving ACR20 for UPA vs PBO at Wk 12. Multiplicity controlled secondary endpoints for each dose of UPA vs PBO included change in HAQ-DI, FACIT-F, and SF-36 PCS (Wk 12); static Investigator Global Assessment of Psoriasis of 0 or 1, PASI75, and change in Self-Assessment of Psoriasis Symptoms (Wk 16); change in modified Sharp/van der Heijde Score (mTSS), proportion of pts achieving MDA, and resolution of enthesitis (LEI=0) and dactylitis (LDI=0) (Wk 24). For each dose of UPA, the multiplicity-controlled analysis also included non-inferiority and superiority vs ADA for ACR20 and superiority for HAQ-DI and pt’s assessment of pain NRS (Wk 12). ACR50/70 at Wk 12 and ACR20 at Wk 2 were additional secondary endpoints. Adverse events (AEs) through 24 wks are reported for pts who received ≥1 dose of study drug.
Results: 1705 pts were randomized; 1704 received study drug (53.2% female, mean age 50.8 yrs, mean duration of PsA diagnosis 6.1 yrs). 82% were on ≥1 concomitant non-bDMARD, of whom 84% received MTX +/- another non-bDMARD.
At Wk 12, ACR20 rates were 70.6% with UPA15 and 78.5% with UPA30 vs 36.2% with PBO (p < .001 for UPA15/30 vs PBO) and 65.0% with ADA (non-inferiority, p < .001 for UPA15/30 vs ADA; superiority, p < .001 for UPA30 vs ADA). A greater proportion of pts achieved ACR50/70 with UPA15/30 vs PBO and UPA30 vs ADA. Improvements were observed with UPA15/30 vs PBO for all multiplicity controlled secondary endpoints and for UPA 15/30 vs ADA for HAQ-DI and UPA 30 vs ADA for improvement in pain (Figure 1A and 1B). At Wk 24, change in mTSS was 0.25 for PBO, -0.04 for UPA15, 0.03 for UPA30, and 0.01 for ADA (p < 0.001 for UPA15/30 vs PBO). AE/SAE rates, including serious infections, were similar in PBO, UPA15, and ADA arms and higher with UPA30 (Figure 2). Herpes zoster rates were similar for PBO and UPA15/30. No MACE was reported with UPA. One malignancy occurred in each PBO and UPA15 arms, and 3 malignancies occurred in each UPA30 and ADA arms. VTE were reported in 1 pt on PBO, 1 pt on UPA30, and 2 pts on ADA. One death occurred in the PBO arm.
Conclusion: In this non-bDMARD-IR PsA population, treatment with UPA15/30 demonstrated improvement in musculoskeletal symptoms, psoriasis, physical function, pain, and fatigue and inhibited radiographic progression; improvements were observed by Wk 2. At Wk 12, UPA15/30 were non-inferior to ADA for ACR20, with superiority demonstrated for UPA30. Greater percentages of UPA vs PBO pts achieved stringent measures of disease control (MDA, ACR50/70, sIGA 0/1). No new safety signals were identified compared with the safety profile observed in RA.
To cite this abstract in AMA style:
McInnes I, Anderson J, Magrey M, Merola J, Liu Y, Kishimoto M, Jeka S, Pacheco Tena C, Wang X, Chen L, Zueger P, Pangan A, Behrens F. Efficacy and Safety of Upadacitinib versus Placebo and Adalimumab in Patients with Active Psoriatic Arthritis and Inadequate Response to Non-Biologic Disease-Modifying Anti-Rheumatic Drugs: A Double-Blind, Randomized Controlled Phase 3 Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-upadacitinib-versus-placebo-and-adalimumab-in-patients-with-active-psoriatic-arthritis-and-inadequate-response-to-non-biologic-disease-modifying-anti-rheumatic-drugs-a-double-b/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-upadacitinib-versus-placebo-and-adalimumab-in-patients-with-active-psoriatic-arthritis-and-inadequate-response-to-non-biologic-disease-modifying-anti-rheumatic-drugs-a-double-b/