Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment I: Psoriatic Arthritis (0504–0508)
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: Upadacitinib (UPA) is an oral, reversible, JAK inhibitor approved for treatment of moderate to severe rheumatoid arthritis (RA) and currently under evaluation for treatment of psoriatic arthritis (PsA). We assess the efficacy and safety of UPA versus placebo (PBO) in patients (pts) with PsA and prior inadequate response or intolerance to ≥1 biologic disease-modifying anti-rheumatic drug (bDMARD).
Methods: In SELECT-PsA-2, pts were randomized 1:1:1 to once daily UPA 15 mg (UPA15), UPA 30 mg (UPA30), or PBO. Pts were stratified by baseline DMARD use, number of prior failed bDMARDs, and extent of psoriasis. The primary endpoint was the proportion of pts achieving ACR20 response at Wk 12. Multiplicity controlled secondary endpoints included change in HAQ-DI, FACIT-Fatigue (FACIT-F), and SF-36 Physical Component Summary (PCS) at Wk 12; static Investigator Global Assessment (sIGA) of Psoriasis of 0 or 1 and at least a 2-point improvement from baseline, PASI75, and change in Self-Assessment of Psoriasis Symptoms (SAPS) at Wk 16; and proportion of pts achieving MDA at Wk 24. Additional key secondary endpoints were ACR50 and ACR70 at Wk 12, and ACR20 at Wk 2. Treatment-emergent adverse events (TEAEs) are reported for pts who received ≥1 dose of study drug.
Results: 641 pts were randomized and received study drug; 54.3% were female with mean age of 53.4 years, and mean duration since PsA diagnosis of 10.1 years. 61% of pts failed 1 bDMARD, 18% failed 2 bDMARDs, and 13% failed ≥3 bDMARDs. 543 (84.6%) pts completed Wk 24 study drug.
At Wk 12, a significantly greater proportion of pts receiving UPA15 and UPA30 vs PBO achieved ACR20 (56.9% and 63.8% vs 24.1%; p < .0001 for both comparisons). Statistically significant improvements were observed in the UPA15 and UPA30 arms vs PBO in all multiplicity controlled secondary endpoints, including ∆HAQ-DI (PBO, -0.10; UPA15, -0.30; UPA30, -0.41), ∆SF-36 PCS (PBO, 1.6; UPA15, 5.2; UPA30, 7.1), ∆FACIT-F (PBO, 1.3; UPA15, 5.0; UPA30, 6.1), and ∆SAPS (PBO, -1.5; UPA15, -24.4; UPA30, -29.7; p < .0001 for all endpoints; Figure 1). In addition, a greater proportion of pts achieved ACR50 and ACR70 at Wk 12 with UPA vs PBO.
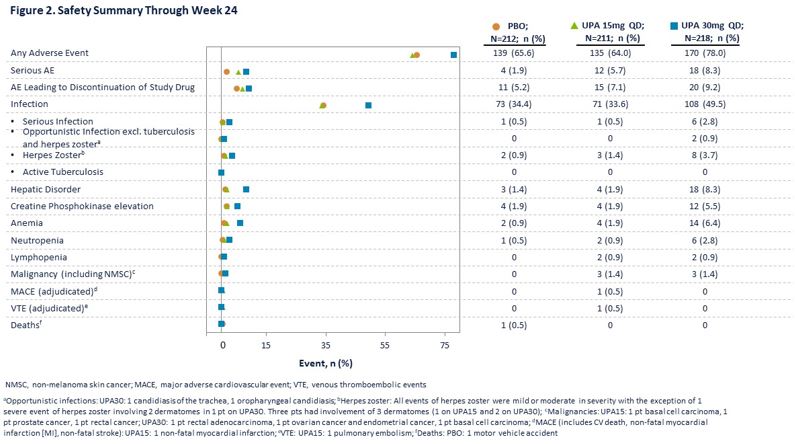
Generally, TEAEs were reported at similar frequencies in the PBO and UPA15 arms and at a higher frequency in the UPA30 arm (Figure 2). Numerically higher rates of serious AEs were reported in the UPA arms. Herpes zoster was more frequent with UPA30. Three malignancies occurred in each of the UPA arms. One adjudicated non-fatal myocardial infarction and one adjudicated pulmonary embolism were reported with UPA15.
Conclusion: In this bDMARD-IR PsA population, UPA15 and UPA30 demonstrated significant improvements across PsA domains including improvements in joint and skin signs and symptoms vs PBO through Wk 24 with improvement observed by Wk 2. A greater percentage of pts treated with UPA achieved MDA and ACR50/70, stringent composite measures of disease control. No new safety signals were identified compared to what has been observed with UPA in RA.
To cite this abstract in AMA style:
Genovese M, Lertratanakul A, Anderson J, Papp K, Tillett W, Van den Bosch F, Tsuji S, Dokoupilova E, Keiserman M, Wang X, Zhong S, Zueger P, Pangan A, Mease P. Efficacy and Safety of Upadacitinib in Patients with Active Psoriatic Arthritis and Inadequate Response to Biologic Disease-Modifying Anti-Rheumatic Drugs: A Double-Blind, Randomized Controlled Phase 3 Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-upadacitinib-in-patients-with-active-psoriatic-arthritis-and-inadequate-response-to-biologic-disease-modifying-anti-rheumatic-drugs-a-double-blind-randomized-controlled-phase/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-upadacitinib-in-patients-with-active-psoriatic-arthritis-and-inadequate-response-to-biologic-disease-modifying-anti-rheumatic-drugs-a-double-blind-randomized-controlled-phase/