Session Information
Date: Sunday, November 7, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster I: Axial Spondyloarthritis (0908–0939)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: The objective of this long-term analysis of the SELECT-AXIS 1 study was to report safety and efficacy of upadacitinib (UPA) in active AS through 2 years.
Methods: SELECT-AXIS 1 (NCT03178487) included a placebo-controlled, 14-week (wk) period followed by a 90-wk open-label extension.1 The study enrolled patients (pts) with active AS (fulfilling modified New York criteria) who had an inadequate response to NSAID therapy and were biologic DMARD naive. At baseline (BL), pts were randomized to UPA 15 mg once daily (QD) or PBO; at wk 14, pts continued to receive UPA 15 mg QD (continuous UPA) while PBO pts were switched to UPA. Efficacy assessments related to signs and symptoms included Assessment of SpondyloArthritis international Society (ASAS) 40 response, ASAS partial remission (PR) and AS Disease Activity Score (ASDAS) inactive disease (ID) and low disease activity (LDA). Imaging assessments included changes from BL in MRI Spondyloarthritis Research Consortium of Canada (SPARCC) spine and SI joint scores and in modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) at wk 104. As observed (AO) and non-responder imputation (NRI) data for binary endpoints and mixed-effect model repeated measures for continuous efficacy endpoints are reported. UPA treatment-emergent adverse events (AEs) were monitored throughout the study.
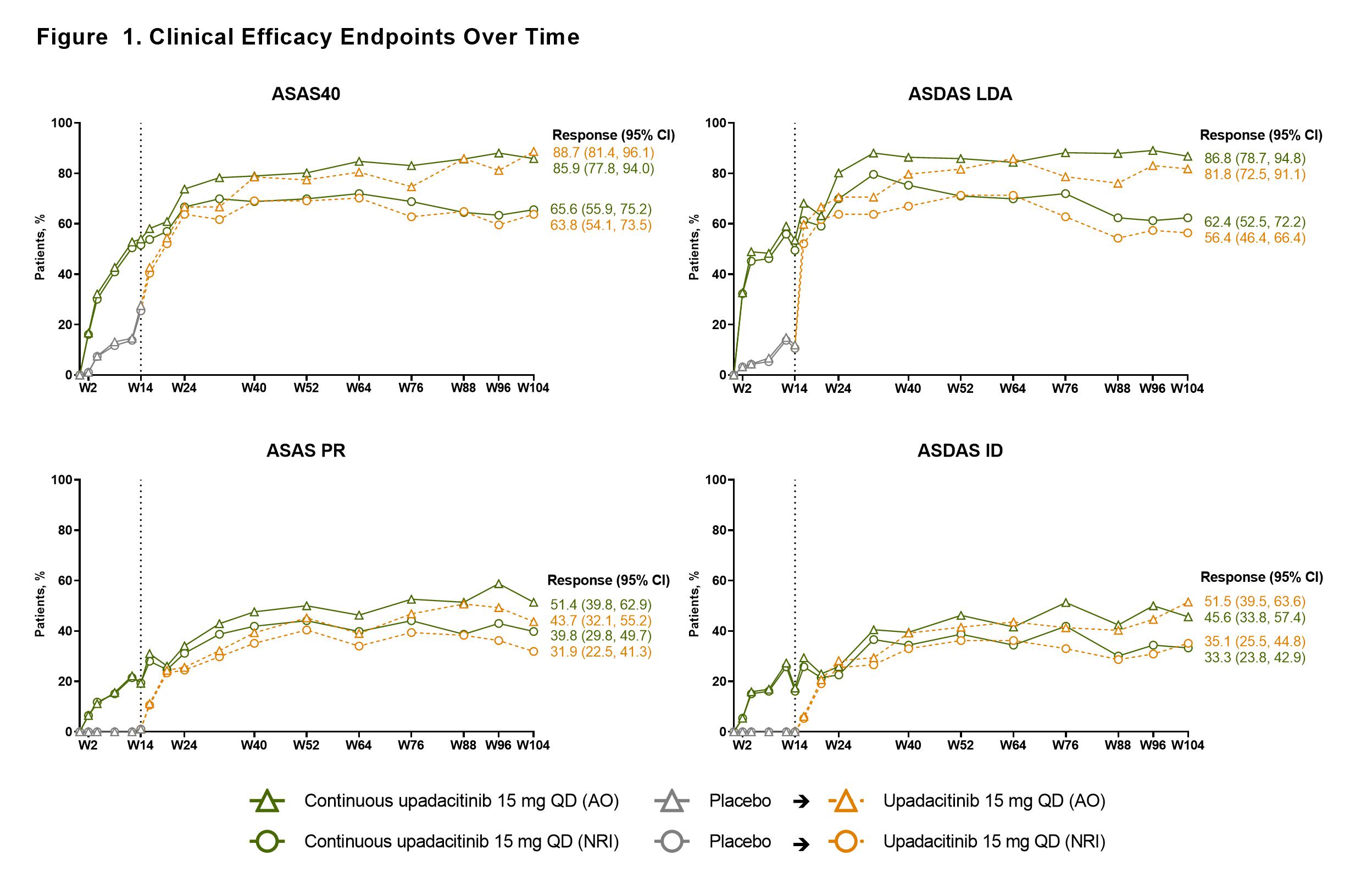
Results: Of 187 pts randomized at BL, 178 pts (each n=89 for UPA and PBO) completed wk 14 on study drug and entered the open-label extension; 144 pts (77%) completed wk 104 (UPA, n=71; PBO to UPA, n=73). ASAS40 response was maintained through 2 years among pts receiving continuous UPA; similar results at wk 104 were observed among pts who switched from PBO to UPA at wk 14 (Figure). A similar pattern and maintenance of response was also observed for other efficacy endpoints (Figure). MRI SPARCC spine and SI joint scores decreased from BL to wk 14 and were maintained thereafter to wk 104 with continuous UPA; a similar magnitude of decrease was seen at wk 104 in pts who switched from PBO to UPA at wk 14 (Table 1). The mean (95% CI) change from BL to wk 104 in the mSASSS was 0.68 (0.27, 1.09) in the total group (Table 1). Overall UPA treatment-emergent AE rate was 242.7/100 patient-years (PY). Infections were the most common AEs; no serious infections, active tuberculosis, adjudicated major adverse cardiovascular events, lymphoma, non-melanoma skin cancer, renal dysfunction, or gastrointestinal perforations were observed. Five (1.6/100 PY) non-serious herpes zoster infections (limited to 1 dermatome), 1 event of colitis (0.3/100 PY), 16 (5.2/100 PY) non-serious uveitis events (mostly in HLA-B27 positive pts with a history of uveitis), and 35 (11.3/100 PY) mostly asymptomatic and transient events of creatine phosphokinase elevation were observed throughout the 2 years among pts receiving UPA (Table 2).
Conclusion: UPA 15 mg QD showed sustained and consistent efficacy over 2 years for ASAS40 (primary endpoint) and other clinically relevant endpoints (ASDAS ID, ASDAS LDA, and ASAS PR). A low rate of radiographic progression was observed based on spinal radiographs. No new safety findings were observed.
1. van der Heijde D, et al. Lancet. 2019;394(10214):2108-2117.
To cite this abstract in AMA style:
van der Heijde D, Deodhar A, Maksymowych W, Sieper J, Van den Bosch F, Kim T, Kishimoto M, Ostor A, Combe B, Sui Y, Duan Y, Chu A, Song I. Efficacy and Safety of Upadacitinib in Patients with Active Ankylosing Spondylitis: 2-Year Results from a Randomized, Double-Blind, Placebo-Controlled Study with Open-Label Extension [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-upadacitinib-in-patients-with-active-ankylosing-spondylitis-2-year-results-from-a-randomized-double-blind-placebo-controlled-study-with-open-label-extension/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-upadacitinib-in-patients-with-active-ankylosing-spondylitis-2-year-results-from-a-randomized-double-blind-placebo-controlled-study-with-open-label-extension/