Session Information
Date: Monday, November 13, 2023
Title: (1554–1578) Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: To compare the efficacy and safety of tofacitinib and tocilizumab in patients with Takayasu arteritis (TAK) in real world situation.
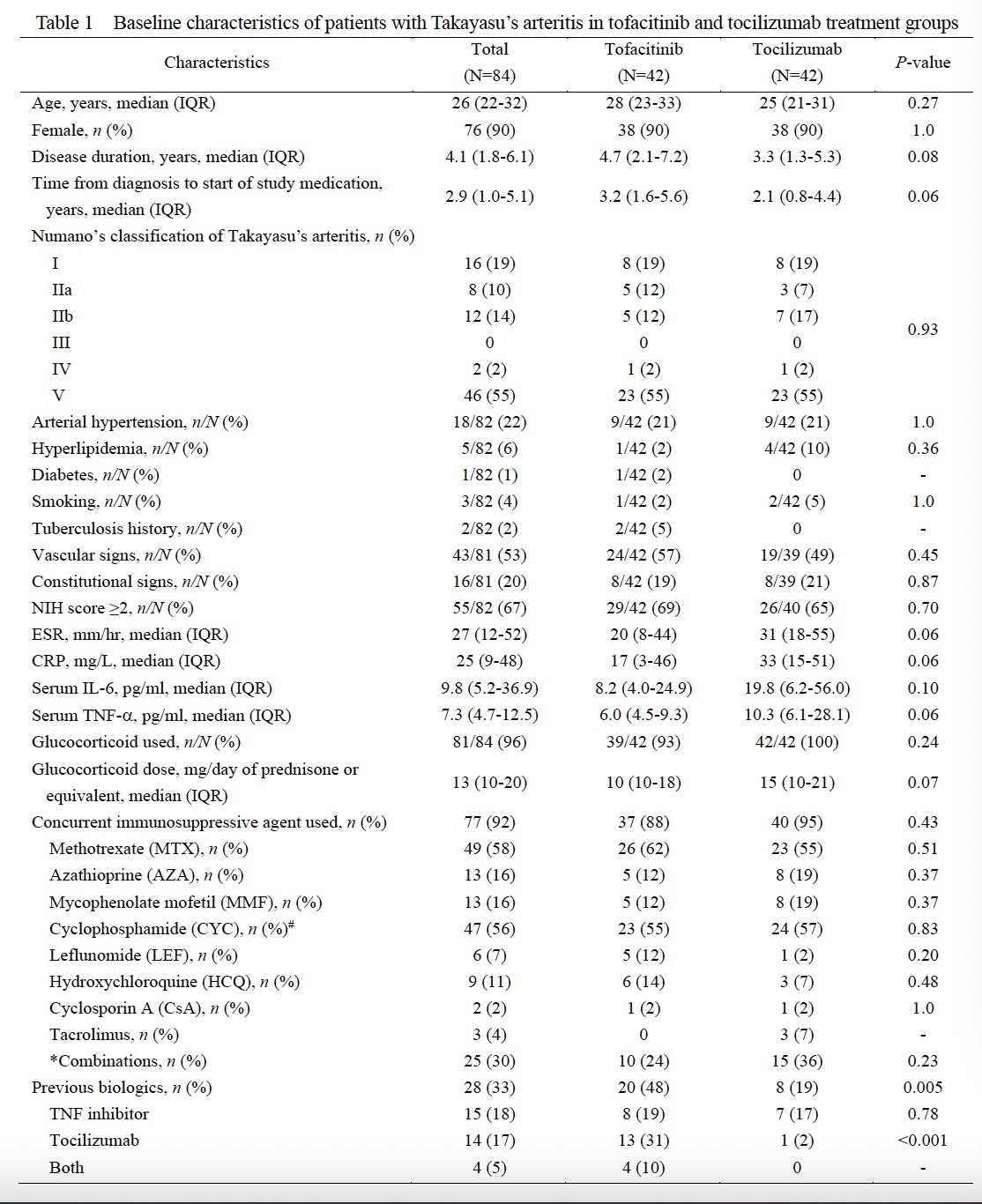
Methods: A post-hoc analysis was conducted in 84 patients with TAK registered in the Chinese Registry of Systemic Vasculitis (CRSV) cohort [median age 26 years (interquartile range 22-32), 76 (90%) females] were included (Figure 1). Patients who were treated with either tofacitinib (n=42) or tocilizumab (n=42) were followed up regularly according to the pre-set protocol. Complete response was defined as both clinically and imagine remission with the dosage of prednisone ≤ 10mg daily and normal acute-phase reactant, while partial response were the same with complete response except that ESR was less than 40mm/h or hs-CRP (hypersensitive C reactive Protein) higher than 16mg/L or the acute-phase reactant decreased by more than 50% compared to the baseline.
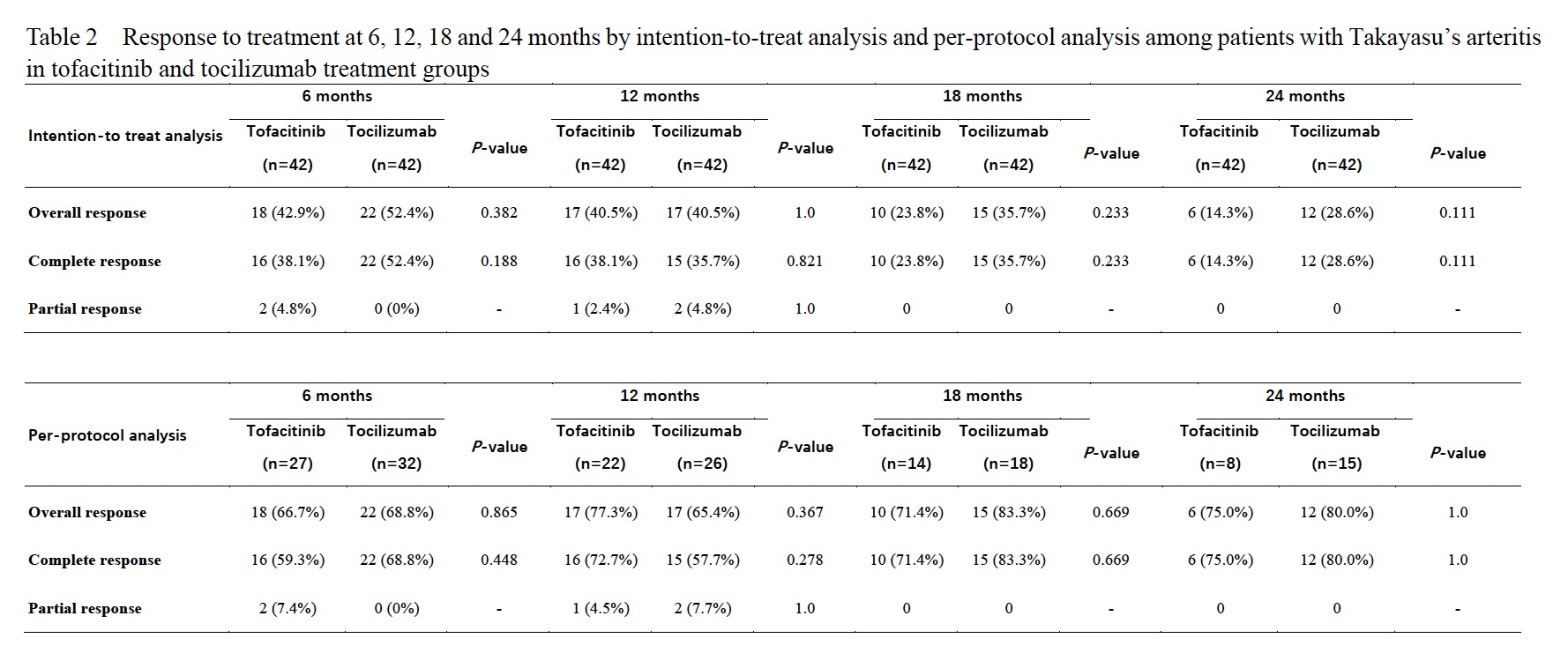
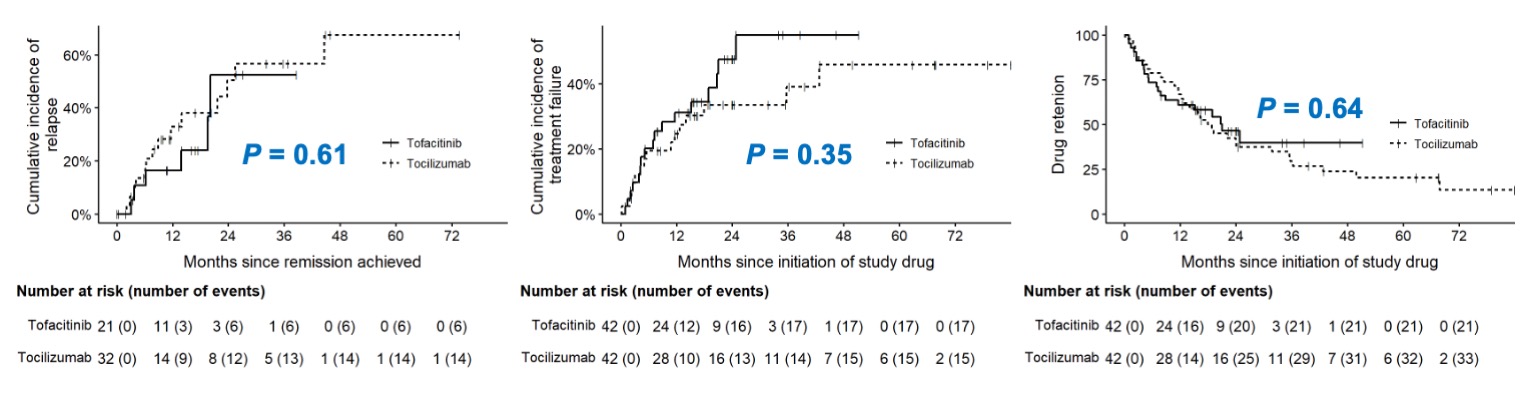
Results: The baseline clinical features and demographic date were similar in these two groups (Table 1). Complete response at 6 months was found in 18/27 (67%) patients treated with tofacitinib and 22/32 (69%) with tocilizumab, and rates of overall response in intention-to-treat (ITT) populations were 43% and 52% respectively (P>0.05). At 12, 18 and 24 months, the rates of overall response in ITT population (41%, 24% and 14% vs. 41%, 36% and 29%) and in per-protocol (PP) population (77%, 71% and 75% vs.65%, 83% and 80%) were similar in patients treated with tofacitinib to those with tocilizumab (Table 2). There were no significant difference found in the changes of ESR, CRP, TNFa, dose of glucocorticoid, mural thickness and lumen diameters of common carotid and subclavian arteries in these two groups. And the cumulative incidences of relapse, treatment failure, and drug retention were similar, too (Figure 1). The drug retention rates at 6, 12, 18 and 24 months in patients treated with tofacitinib and tocilizumab were 64%, 52%, 33% and 19% vs. 76%, 60%, 43% and 36%, respectively.
Six (14%) adverse events occurred in the tofacitinib treatment group (including 3 patients with herpes zoster infection, 1 with pulmonary infection, 1 with headache and 1 with abdominal pain) and 6 (14%) occurred in the tocilizumab treatment group (including 2 patients with back pain, 1 with acute pancreatitis, 1 with pulmonary infection, 1 with vomiting and 1 with hepatic dysfunction).
Conclusion: Tofacitinib and tocilizumab are both effective for refractory TAK with similar response rates, cumulative incidences of relapse, treatment failure, and drug retention. Both medications may be effective alternatives for patients who failed to respond to traditional immunosuppressive agents.
* Combinations of immunosuppressive agents: MTX+MMF(7), MTX+AZA(2), MMF+HCQ(2), LEF+HCQ(2), MTX+TAC(1), MTX+LEF(1), MTX+HCQ(1), MTX+CTX(1), MTX+CsA(1), MTX+HCQ+CsA(1), MTX+HCQ+AZA(1), CTX+TAC(1), AZA+TAC(1), AZA+LEF(1), AZA+HCQ(1), AZA+CTX(1).
# Number of patients who ever received cyclophosphamide.
To cite this abstract in AMA style:
Li J, Zhao Y, Yu Y, Jian X, Wang Y, yang y, Tian X, Li M, Zeng X. Efficacy and Safety of Tofacitinib and Tocilizumab in 84 Patients with Takayasu Arteritis: Single-Center Post-Hoc Analysis of a Prospective Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-tofacitinib-and-tocilizumab-in-84-patients-with-takayasu-arteritis-single-center-post-hoc-analysis-of-a-prospective-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-tofacitinib-and-tocilizumab-in-84-patients-with-takayasu-arteritis-single-center-post-hoc-analysis-of-a-prospective-study/