Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Glucocorticoids (GC) are the mainstay of treatment options for patients (pts) with Takayasu arteritis (TAK); however, long-term GC therapy is associated with adverse events (AEs). TAK pts have elevated IL-6 levels, which correlates with TAK disease activity (Rheumatology. 2006;45:545-48). Tocilizumab (TCZ), a humanized anti–IL-6 receptor antibody, was investigated for the treatment of relapsing TAK.
Methods: Pts ≥12 y with TAK (Circ J. 2011;75:474-503) receiving GC (≥0.2 mg/kg/d prednisolone equivalent) who had relapsed ≤12 weeks (wks) before enrollment were randomly assigned 1:1 to subcutaneous (SC) TCZ 162 mg or placebo (PBO) every wk (QW). Pts had to be receiving a stable GC dose at ≥2× dose at relapse and to be in remission for 1 wk before randomization. During the double-blind (DB) period, which lasted until relapse occurred in 19 pts, background GC were tapered by 10%/wk from wk 4, which is more rapid than in a general clinical setting. The primary end point was time to first relapse of TAK per protocol-defined criteria in the intent-to-treat (ITT) population estimated with the Kaplan-Meier analysis method and analyzed with the log-rank test stratified by age (<18 y, 18–64 y, ≥65 y). Time to relapse by Kerr’s definition (Ann Intern Med. 1994;120:919-29) was a secondary end point.
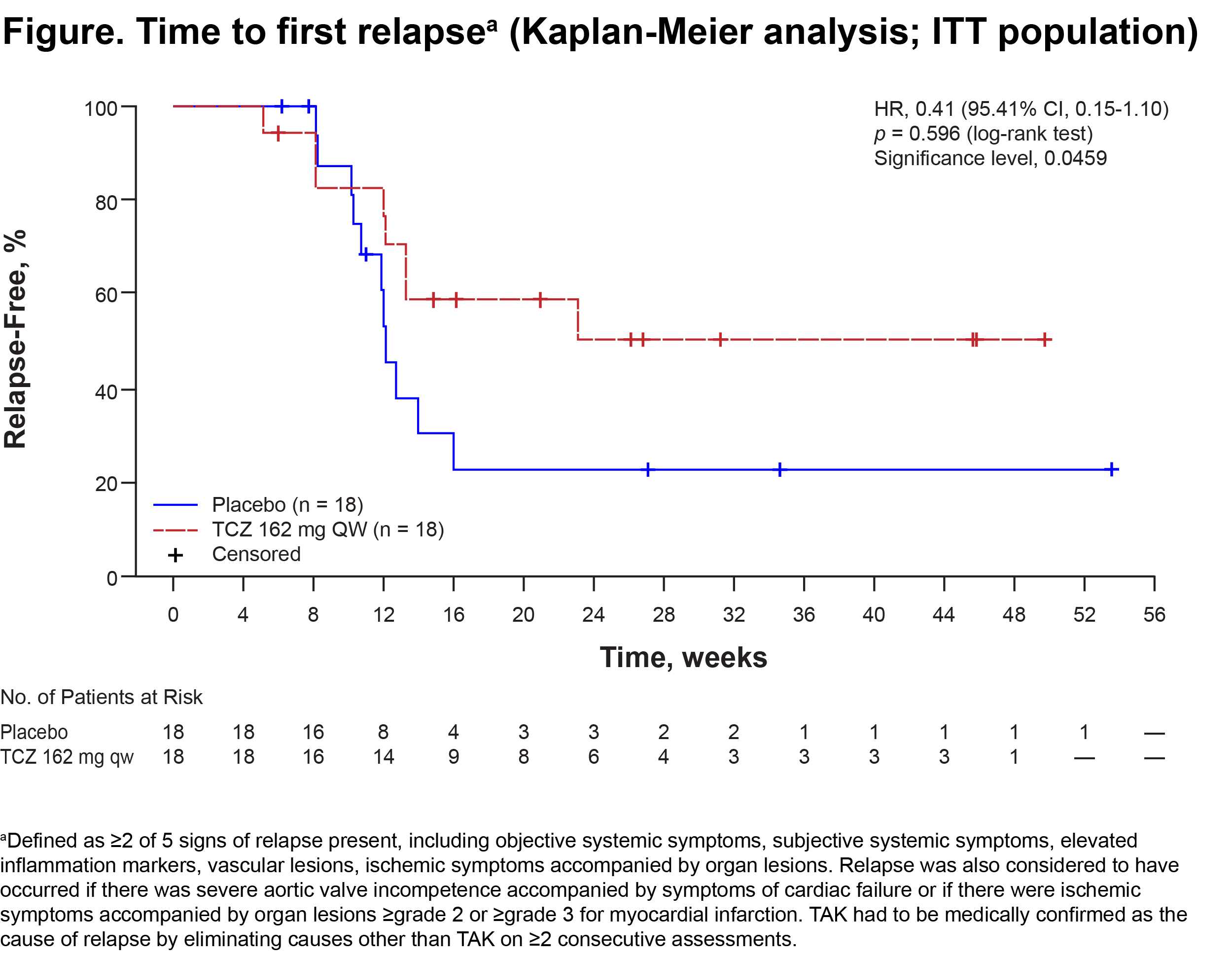
Results: The ITT and safety populations included 18 TCZ pts and 18 PBO pts; median disease duration was 3.33 y and 2.89 y, respectively; mean ± SD GC dose at randomization was 0.57 ± 0.19 (TCZ) and 0.52 ± 0.16 (PBO) mg/kg/d; 38.9% of TCZ and 72.2% of PBO pts were HLA-B52 positive; 86.1% of pts were female. The per-protocol (PP) population included 16 TCZ and 17 PBO pts. No pts withdrew during the DB period. In the ITT population, 8 (44.4%) TCZ and 11 (61.1%) PBO pts relapsed. Estimated relapse-free rates at wk 24 were 50.6% and 22.9%, respectively, no statistical difference of time to first relapse between groups was seen (Figure; hazard ratio [HR], 0.41 [95.41% CI, 0.15-1.10]; p = 0.0596). Results were the same with Kerr’s definition. In the PP population, for relapse according to protocol-defined criteria, HR was 0.34 and p = 0.0345 (95.41% CI, 0.11-1.00), favoring TCZ. In addition, TCZ showed favorable trends in each sign of relapse, including objective systemic symptoms, subjective systemic symptoms, elevated inflammation markers, vascular lesions, and ischemic symptoms accompanied by organ lesions (ITT population). AEs were reported in 14 (77.8%) TCZ and 11 (61.1%) PBO pts, and serious AEs were reported in 1 pt and 2 pts. Infections were the most frequent AEs. No pts died.
Conclusion: There was a trend toward relapse suppression favoring TCZ, though the primary end point was not met. The safety of TCZ was consistent with the current safety profile for TCZ in RA/JIA. TCZ may be a promising treatment option for rapidly tapering GC in TAK pts.
To cite this abstract in AMA style:
Nakaoka Y, Isobe M, Takei S, Tanaka Y, Ishii T, Yokota S, Nomura A, Yoshida S, Nishimoto N. Efficacy and Safety of Tocilizumab in Patients with Refractory Takayasu Arteritis: Results from a Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial in Japan [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-tocilizumab-in-patients-with-refractory-takayasu-arteritis-results-from-a-randomized-double-blind-placebo-controlled-phase-3-trial-in-japan/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-tocilizumab-in-patients-with-refractory-takayasu-arteritis-results-from-a-randomized-double-blind-placebo-controlled-phase-3-trial-in-japan/