Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Efficacy and safety of tocilizumab (TCZ), an interleukin-6 receptor inhibitor, were previously demonstrated at week 40 of CHERISH, a phase 3 trial in patients with polyarticular-course juvenile idiopathic arthritis (pcJIA).1 The purpose of this analysis was to investigate the efficacy and safety of TCZ over 104 weeks of treatment in pcJIA.
Methods: Patients 2-17 years old with ≥6 months’ active pcJIA who failed methotrexate received open-label (OL) TCZ (weight ≥30 kg, 8 mg/kg [n=119]; weight <30 kg, randomized [1:1] to 8 [n=34] or 10 [n=35] mg/kg) every 4 weeks for 16 weeks. Patients with JIA American College of Rheumatology (ACR)30 response or better at week 16 entered a 24-week double-blind withdrawal period and were randomized (1:1) to placebo or continuation with TCZ. Patients with JIA ACR30 flare or who completed the withdrawal period entered an OL extension through week 104.
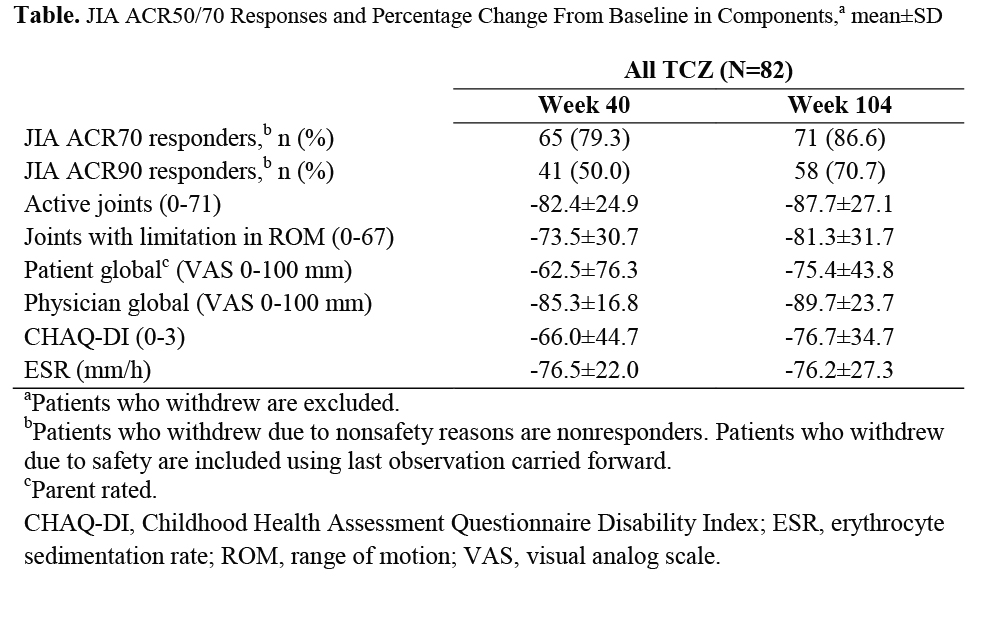
Results: One hundred eighty-eight patients entered the lead-in period, 166 entered the withdrawal period, 160 entered the OL extension period, and 155 completed 104 weeks. In patients who received TCZ throughout the study, JIA ACR responses and improvement in JIA ACR core components (Table) were maintained through week 104. The safety population comprised 188 patients with 307 patient-years (PY). Rates/100PY of adverse events (AEs) and serious AEs (SAEs) were 406.5 and 11.1, respectively; infections were the most common AE (151.4) and SAE (5.2). Alanine aminotransferase and aspartate aminotransferase elevations ≥3× upper limit of normal occurred in 6.4% and 2.7% of patients, respectively. Grade 3 neutropenia and grade 2/3/4 thrombocytopenia occurred in 5.9% and 1.6% of patients, respectively. Low-density lipoprotein cholesterol ≥110 mg/dL occurred in 16.2% of patients.
Conclusion: Efficacy of TCZ was maintained through 2 years of treatment in patients with pcJIA, with no change in safety profile from that reported previously.1
Reference: 1. Brunner H et al. Arthritis Rheum 2012;64:2012.
Disclosure:
H. I. Brunner,
Novartis, Genentech, MedImmune, EMD Serono, AMS, Pfizer, UCB, Janssen,
5,
Genentech ,
8;
N. Ruperto,
Abbott, AstraZeneca, BMS, Centocor, Lilly, Francesco Angelini, GSK, Italfarmaco, MerckSerono, Novartis, Pfizer, Regeneron, Roche, Sanofi Aventis, Schwarz Biosciences, Xoma, Wyeth,
2,
Abbott, AstraZeneca, BMS, Centocor, Lilly, Francesco Angelini, GSK, Italfarmaco, MerckSerono, Novartis, Pfizer, Regeneron, Roche, Sanofi Aventis, Schwarz Biosciences, Xoma, Wyeth,
5,
Abbott, Boehringer, BMS, Novartis, Astellas, Italfarmaco, MedImmune, Pfizer, Roche,
8;
Z. Zuber,
None;
R. J. Cuttica,
Roche, Abbott, Pfizer, Novartis, BMS,
8;
R. Xavier,
None;
I. Calvo,
None;
N. Rubio,
None;
E. Alekseeva,
Roche, Abbott, Pfizer, BMS, Centocor, Novartis,
2,
Roche, Merck, Abbott, BMS, Medac, Novartis, Pfizer,
8;
V. Chasnyk,
None;
J. Chavez,
None;
G. Horneff,
Abbott, Pfizer,
2;
V. Opoka-Winiarska,
None;
P. Quartier,
Abbvie, Chugai-Roche, Novartis, Pfizer,
2,
Abbvie, BMS, Chugai-Roche, Novartis, Pfizer, Servier, Sweedish Orphan Biovitrum,
5,
Chugai-Roche, Novartis, Pfizer,
8;
A. Spindler,
None;
C. Keane,
Roche Pharmaceuticals,
3;
K. N. Bharucha,
Genentech and Biogen IDEC Inc.,
3;
J. Wang,
Roche Pharmaceuticals,
3;
D. J. Lovell,
NIH,
2,
AstraZeneca, Centocor, Janssen, Wyeth, Amgen, Bristol-Meyers Squibb, Abbott, Pfizer, Regeneron, Hoffmann-La Roche, Novartis, Genentech,
5,
Roche, Genentech,
8;
A. Martini,
Abbott, AstraZeneca, BMS, Centocor, Lilly, Francesco Angelini, GSK, Italfarmaco, MerckSerono, Novartis, Pfizer, Regeneron, Roche, Sanofi Aventis, Schwarz Biosciences, Xoma, Wyeth,
2,
Abbott, AstraZeneca, BMS, Centocor, Lilly, Francesco Angelini, GSK, Italfarmaco, MerckSerono, Novartis, Pfizer, Regeneron, Roche, Sanofi Aventis, Schwarz Biosciences, Xoma, Wyeth,
5,
Abbott, Boehringer, BMS, Novartis, Astellas, Italfarmaco, MedImmune, Pfizer, Roche,
8;
F. De Benedetti,
Abbott, Pfizer, BMS, Roche, Novimmune, Novartis, SOBI,
2.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-tocilizumab-in-patients-with-polyarticular-course-juvenile-idiopathic-arthritis-2-year-data-from-cherish/