Session Information
Date: Monday, November 14, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Baricitinib (bari) is an oral JAK1/JAK2 inhibitor under investigation for the treatment of patients (pts) with moderate to severe RA.1-2 In the 52-week Phase 3 RA-BEAM study, bari 4 mg once daily (QD) showed clinical improvements compared to placebo (PBO) and to adalimumab (ADA) in MTX-inadequate-responder (IR) pts.2 The objective of this analysis was to evaluate efficacy and safety in pts from RA-BEAM who changed treatment from ADA to bari either after rescue in RA-BEAM or switch after entering a long-term extension (LTE) study (RA-BEYOND).
Methods: In RA-BEAM (completed September 2015), 1305 pts were randomized 3:3:2 to PBO, bari 4 mg QD, or ADA 40 mg every 2 weeks (wks). At Wk 16 or subsequent visits, IRs (lack of ≥20% reduction in tender and swollen joint count) were rescued to open-label bari 4 mg. At Wk 52, pts could enter the LTE, where all pts received bari 4 mg and remained blinded to their randomized treatment in RA-BEAM. No ADA washout period was applied for rescue or switch from ADA to bari. Efficacy analyses evaluated both rescued and not rescued RA-BEAM pts who entered the LTE ≥24 wks before the present data cutoff. Safety analyses included pts not rescued in RA-BEAM who entered the LTE.
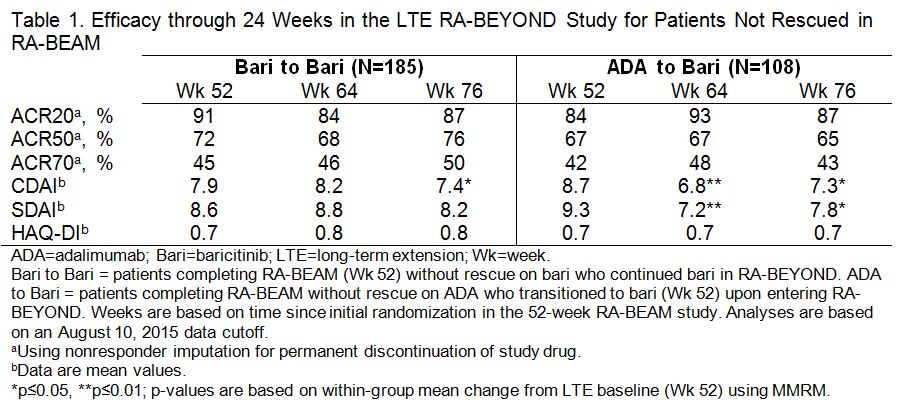
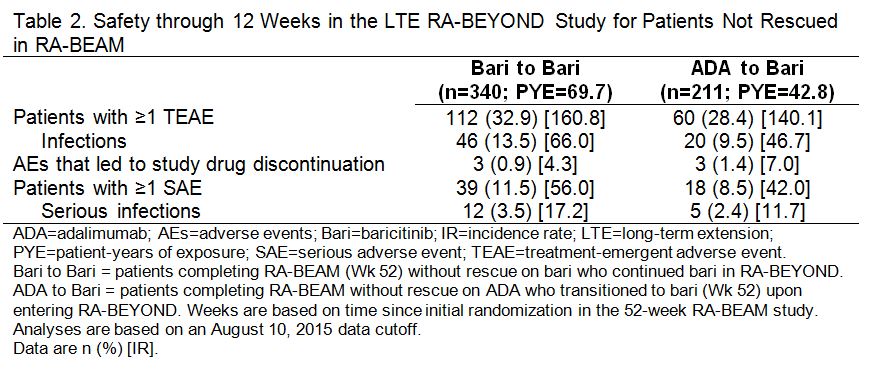
Results: A total of 51 pts were rescued from ADA to bari 4 mg in RA-BEAM; at Wk 52, 67%, 49%, and 24% achieved ACR20, ACR50, and ACR70, respectively. Among pts who completed RA-BEAM without rescue, 381/394 (97%) bari, and 238/241 (99%) ADA pts entered the LTE. Of these, 185 (continued bari) and 108 ADA (switched to bari) pts reached the 24 wk time point and were included in the LTE efficacy analysis and 340 (continued bari) and 211 ADA (switched to bari) pts were included in the LTE safety analysis. Patients who switched from ADA to bari showed improvements in disease control through 12 wks post-switch in the LTE, without evidence of worsening through the following 12 wks (Table 1). Exposure-adjusted incidence rates for total treatment-emergent adverse events (TEAEs) and infections, including serious events, were similar for pts who switched from ADA to bari and those who continued on bari (Table 2).
Conclusion: Switching from ADA to bari without ADA washout was associated with improvements in disease control during the initial 12 wks post-switch, without an increase in overall TEAEs or serious AEs or infections, and without subsequent evidence of worsening. References: 1Dougados M et al. Ann Rheum Dis 2015;74(S2):79. 2Taylor PC et al. Arthritis Rheumatol 2015;67(S10):3927-3928.
To cite this abstract in AMA style:
Taylor PC, Keystone E, Ortmann R, Issa M, Xie L, Muram D, Bradley JD, de Bono S, Rooney T, Tanaka Y. Efficacy and Safety of Switching from Adalimumab to Baricitinib: Phase 3 Data in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-switching-from-adalimumab-to-baricitinib-phase-3-data-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-switching-from-adalimumab-to-baricitinib-phase-3-data-in-patients-with-rheumatoid-arthritis/