Session Information
Date: Monday, November 9, 2015
Title: Rheumatoid Arthritis - Small Molecules, Biologics and Gene Therapy Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
Pharmacokinetic (PK) equivalence and
similarity of clinical efficacy, safety and immunogenicity up to week 24 were demonstrated
between CT-P10, a biosimilar candidate for innovator rituximab (RTX), and RTX
groups in patients with rheumatoid arthritis (RA) 1.
The objective of this open-label study was to confirm efficacy and safety of switched
CT‑P10 from RTX in RA patients (NCT01873443).
Methods :
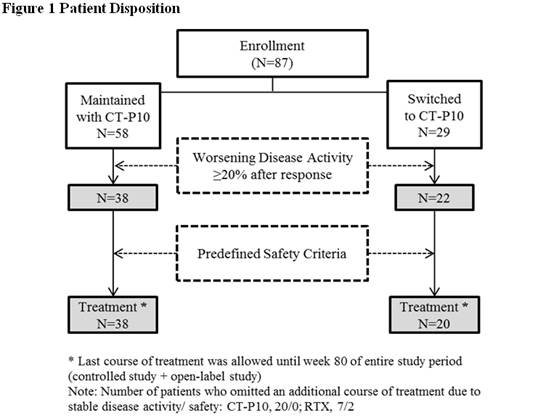
A total of 87 patients,
who
completed up to 72 weeks of the phase I randomized controlled trial
(NCT01534884), entered into the open-label extension study for maximum of 56
weeks: 58 and 29 patients were recruited from CT-P10 and RTX groups,
respectively, in the controlled study. Thirty eight (65.5%) and 20 (69.0%)
patients in each group received CT-P10 treatment according to DAS28 and the
predefined safety criteria during this open-label study (Figure 1). Among 29
patients who did not receive CT-P10 treatment during the study, disease activity
was well controlled until the end of study in 20 and 7 patients from the CT‑P10
and RTX groups, respectively. Two patients in RTX group did not receive CT-P10
treatment due to safety reason. Efficacy and safety assessments were monitored
throughout the study.
Results :
The DAS28-CRP and ESR improvement at Week 24 after
the last CT-P10 infusion were similar in the 2 treatment groups; -2.2 for both
groups in DAS28-CRP (p=0.9474) and -2.7 for maintained CT-P10 group and -2.4
for switched CT-P10 group in DAS28-ESR (p=0.5687).
The proportion of patients experienced at least one adverse event (AE) or serious
AE was comparable between maintained and switched CT-P10 groups. Infusion
related reactions were reported in 1 patient in each treatment group (Table 1).
No
deaths, malignancy or AEs leading to permanent study drug discontinuation were
reported during the study.
Table
1
Efficacy by DAS28 Changes
|
|
Maintained CT-P10 Group |
Switched CT-P10 Group |
|
DAS28-CRP, Mean ± SD (n) |
|
|
|
Baseline |
5.9 ± 0.90 (38) |
5.8 ± 0.72 (18) |
|
Changes at Week 24 after 1st Course |
-2.2 ±1.15 (33) |
-2.2 ± 1.16 (16) |
|
DAS28-ESR, Mean ± SD (n) |
|
|
|
Baseline |
6.8 ±0.83 (38) |
6.5 ±0.80 (18) |
|
Changes at Week 24 after 1st Course |
-2.7 ±1.17 (34) |
-2.4 ±1.33 (16) |
RTX, innovator
rituximab; DAS28, disease activity score in 28 joints; CRP, C-reactive protein;
ESR, erythrocyte sedimentation rate; SD, standard deviation
Table
2
Safety Summary
|
|
Maintained CT-P10 Group N=38 |
Switched CT-P10 Group N=20 |
Total N=58 |
|
Number of Patients (%) with at Least One |
|||
|
AE |
9 (23.7) |
4 (20.0) |
13 (22.4) |
|
Serious AE |
1 (2.6) |
1 (5.0) |
2 (3.4) |
|
Infusion-related reaction |
1 (2.6) |
1 (5.0) |
2 (3.4) |
|
Infection |
3 (7.9) |
2 (10.0) |
5 (8.6) |
|
Malignancy |
0 |
0 |
0 |
|
Discontinuation due to AEs |
0 |
0 |
0 |
RTX, innovator rituximab; AE, adverse events
Conclusion :
Switched CT-P10 from RTX demonstrated comparable efficacy and safety profiles
compared to those of maintained CT-P10. Maintained CT-P10
was also well tolerated and effective up to
2 years in RA patients.
Reference
1 Yoo
DH,
et al.
Arthritis
Rheum 2013; 65 (Suppl
10):
S736
To cite this abstract in AMA style:
Yoo DH, Park W, Suh CH, Shim SC, Cons Molina F, Jeka S, Brzezicki J, Medina-Rodriguez FG, Hrycaj P, Wiland P, Lee EY, Shesternya P, Kovalenko V, Myasoutova L, Stanislav M, Radominski S, Lim MJ, Choe JY, Lee SY, Lee SJ. Efficacy and Safety of Switched CT-P10 from Innovator Rituximab Compared to Those of Maintained CT-P10 in Patients with Rheumatoid Arthritis up to 56 Weeks [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-switched-ct-p10-from-innovator-rituximab-compared-to-those-of-maintained-ct-p10-in-patients-with-rheumatoid-arthritis-up-to-56-weeks/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-switched-ct-p10-from-innovator-rituximab-compared-to-those-of-maintained-ct-p10-in-patients-with-rheumatoid-arthritis-up-to-56-weeks/