Background/Purpose: Disease control remains an unmet need in SLE. The rationale for sequential BEL and RTX therapy in SLE was previously published.1 This study evaluated the efficacy, safety, and tolerability of BEL combined with a single RTX cycle in patients (pts) with SLE, using novel disease control/remission endpoints.
Methods: In this Phase 3, double-blind, placebo (PBO)-controlled, 104-week (wk) study (NCT03312907), pts with active SLE were randomized (1:2:1) to: subcutaneous (SC) BEL 200 mg/wk for 52 wks + intravenous (IV) PBO at Wks 4 and 6 (BEL/PBO); BEL 200 mg/week SC for 52 wks + RTX 1000 mg IV at Wks 4 and 6 (BEL/RTX); or BEL 200 mg/wk SC + standard therapy (ST) for 104 wks (BEL/ST). The 52-wk treatment phase (BEL/PBO and BEL/RTX) was followed by a 52-wk observational phase. The primary efficacy endpoint was the proportion of pts with disease control (SLEDAI-2K score 2 without other immunosuppressants and prednisone-equivalent dose of ≤5 mg/day) at Wk 52. Primary comparison was BEL/RTX vs BEL/PBO; BEL/ST was included for reference and exploratory comparison. Major secondary efficacy endpoints included proportion of pts in clinical remission (clinical SLEDAI-2K=0, without other immunosuppressants and corticosteroids) at Wk 64 and with disease control at Wk 104. Other endpoints included disease control duration, LLDAS, anti-dsDNA, and safety. Overall type 1 error rate was controlled for primary and major secondary endpoints, but not for other endpoints.
Results: Of 396 screened pts, 292 were randomized and received ≥1 study treatment dose (BEL/PBO n=72, BEL/RTX n=144, BEL/ST n=76; intention-to-treat [ITT] population). Most were female (91.8%); mean (SD) age was 40.5 (12.0) years, with a mean (SD) SLEDAI-2K score at baseline (BL) of 10.3 (3.8). At BL, 61.1% of BEL/PBO, 66.0% of BEL/RTX, and 57.9% of BEL/ST pts were anti-dsDNA positive (≥30 IU/ml). Overall, 19.4%, 18.8%, and 19.7% of pts in BEL/PBO, BEL/RTX, and BEL/ST groups, respectively, discontinued BEL study treatment by Wk 52, mainly owing to adverse events (AE) and withdrawal by pt.
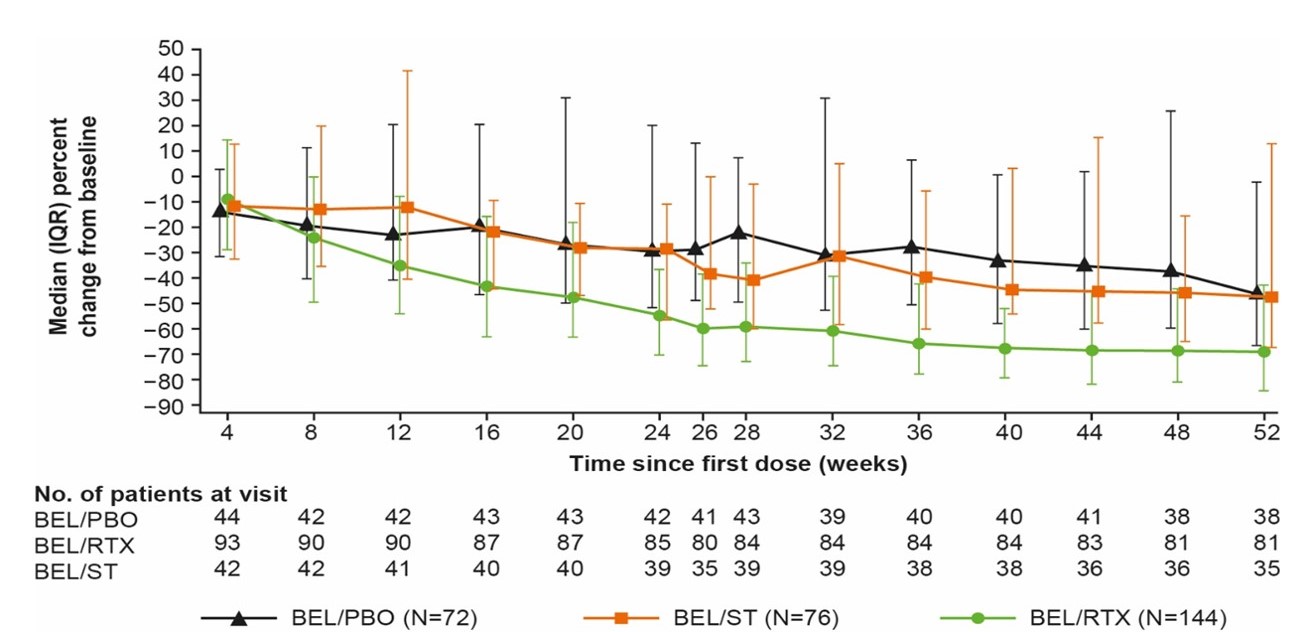
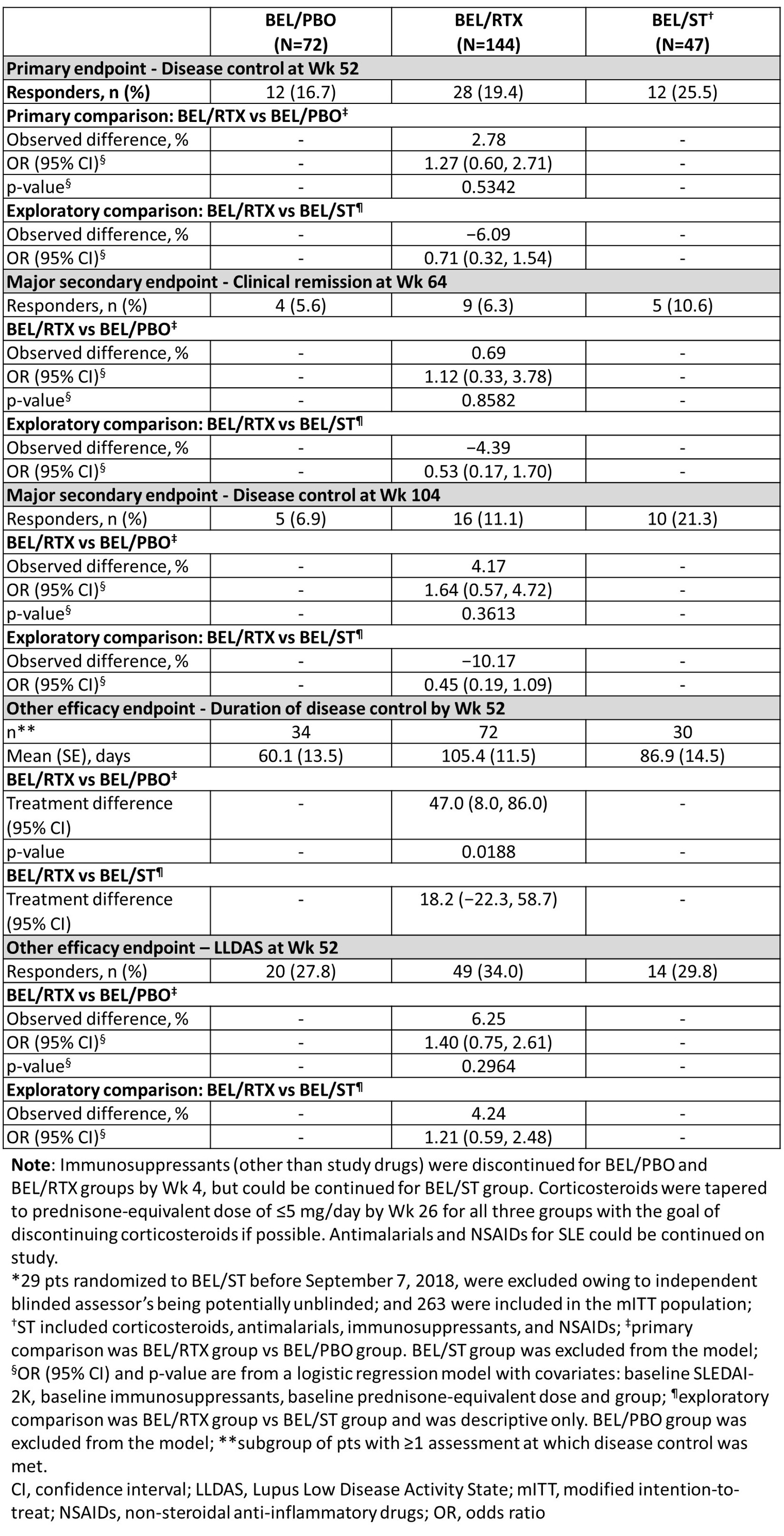
The differences in the proportions of pts with disease control at Wk 52, clinical remission at Wk 64, and disease control at Wk 104 were not statistically significant for BEL/RTX vs BEL/PBO (Table 1). Other efficacy endpoints through Wk 52 were not significantly different for BEL/RTX vs BEL/PBO, except for disease control duration by Wk 52 (Table 1). At Wk 52, in pts who were anti-dsDNA positive at BL, a significantly greater decrease from BL in anti-dsDNA was observed with BEL/RTX vs BEL/PBO (Figure 1).
AE incidence by Wk 52 was similar across groups; however, more AEs resulted in treatment discontinuation/withdrawal in BEL/RTX vs BEL/PBO or BEL/ST. More serious AEs were reported with BEL/RTX vs BEL/PBO or BEL/ST, mainly due to serious infections/infestations. AEs of special interest showed no imbalance between BEL/RTX vs BEL/PBO (Table ).
Conclusion: Adding a single cycle of RTX to BEL did not improve disease control/remission. Safety findings were consistent with known BEL and RTX safety profiles, with more serious infections observed in pts treated with BEL/RTX vs BEL/PBO or BEL/ST
1. Onno Teng YK, et al. BMJ Open 2019;9(3):e025687.
To cite this abstract in AMA style:
Aranow C, Allaart C, Amoura Z, Bruce I, Cagnoli P, Furie R, Tak P, Urowitz M, van Vollenhoven R, Clark K, Daniels M, Fox N, Gregan Y, Groark J, Henderson R, Oldham M, Shanahan D, van Maurik A, Roth D, Teng Y. Efficacy and Safety of Subcutaneous Belimumab (BEL) and Rituximab (RTX) Sequential Therapy in Patients with Systemic Lupus Erythematosus: The Phase 3, Randomized, Placebo-Controlled BLISS-BELIEVE Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-subcutaneous-belimumab-bel-and-rituximab-rtx-sequential-therapy-in-patients-with-systemic-lupus-erythematosus-the-phase-3-randomized-placebo-controlled-bliss-believe-stud/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-subcutaneous-belimumab-bel-and-rituximab-rtx-sequential-therapy-in-patients-with-systemic-lupus-erythematosus-the-phase-3-randomized-placebo-controlled-bliss-believe-stud/