Session Information
Date: Monday, October 27, 2025
Title: (1147–1190) Miscellaneous Rheumatic & Inflammatory Diseases Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Macrophage activation syndrome (MAS) is a rare and life-threatening complication of connective tissue diseases (CTDs), with approximately 30% of cases being refractory to standard therapeutic protocols. Ruxolitinib, a Janus kinase 1/2 inhibitor, has emerged as a potential therapeutic option for refractory MAS. This study aimed to evaluate its efficacy and safety in patients with refractory CTD-associated MAS (CTD-MAS).
Methods: A meticulous chart review was conducted on 20 patients with refractory CTD-MAS who received ruxolitinib in our institution. Data from ruxolitinib-naïve CTD-MAS patients served as historical controls. Clinical and laboratory parameters, therapeutic response, and survival outcomes were analyzed. The efficacy and safety of ruxolitinib were evaluated, and survival rates were compared to historical controls.
Results: The cohort of 20 patients with refractory CTD-MAS included 17 females and 3 males, with underlying conditions comprising adult-onset Still’s disease (n = 13), systemic lupus erythematosus (n = 4), and other connective tissue diseases (n = 3) (Table 1). At the initiation of ruxolitinib treatment, all patients exhibited active diseases. The survival rate was significantly higher in the ruxolitinib group (19/20, 95%) than in historical controls (13/21, 62%) (P = 0.011) (Figure 1). Partial response (PR) was observed in 9 patients at the first scheduled response assessment (week 1), and the first complete remission (CR) was documented at week 2. Two additional patients progressed from PR to CR during the third week, and one patient improved from no response (NR) to PR. By week 8, 50% (10/20) and 30% (6/20) of ruxolitinib-treated patients had achieved partial and complete responses, respectively. Four patients exhibited no response to treatment, including the patient who died of complications (Figure 2). Ruxolitinib therapy led to rapid and significant clinical and laboratory parameter improvements. By week 8, the median daily glucocorticoid dose decreased from 2.7 mg/kg/day to 0.5 mg/kg/day. Cytomegalovirus infection occurred in 20% (4/20) of patients.
Conclusion: Ruxolitinib demonstrated substantial efficacy and tolerability in patients with refractory CTD-MAS, leading to significant clinical improvement and a notable reduction in glucocorticoid dependence. These findings support the potential role of ruxolitinib as a salvage therapy for refractory CTD-MAS, warranting further investigation in prospective clinical studies.
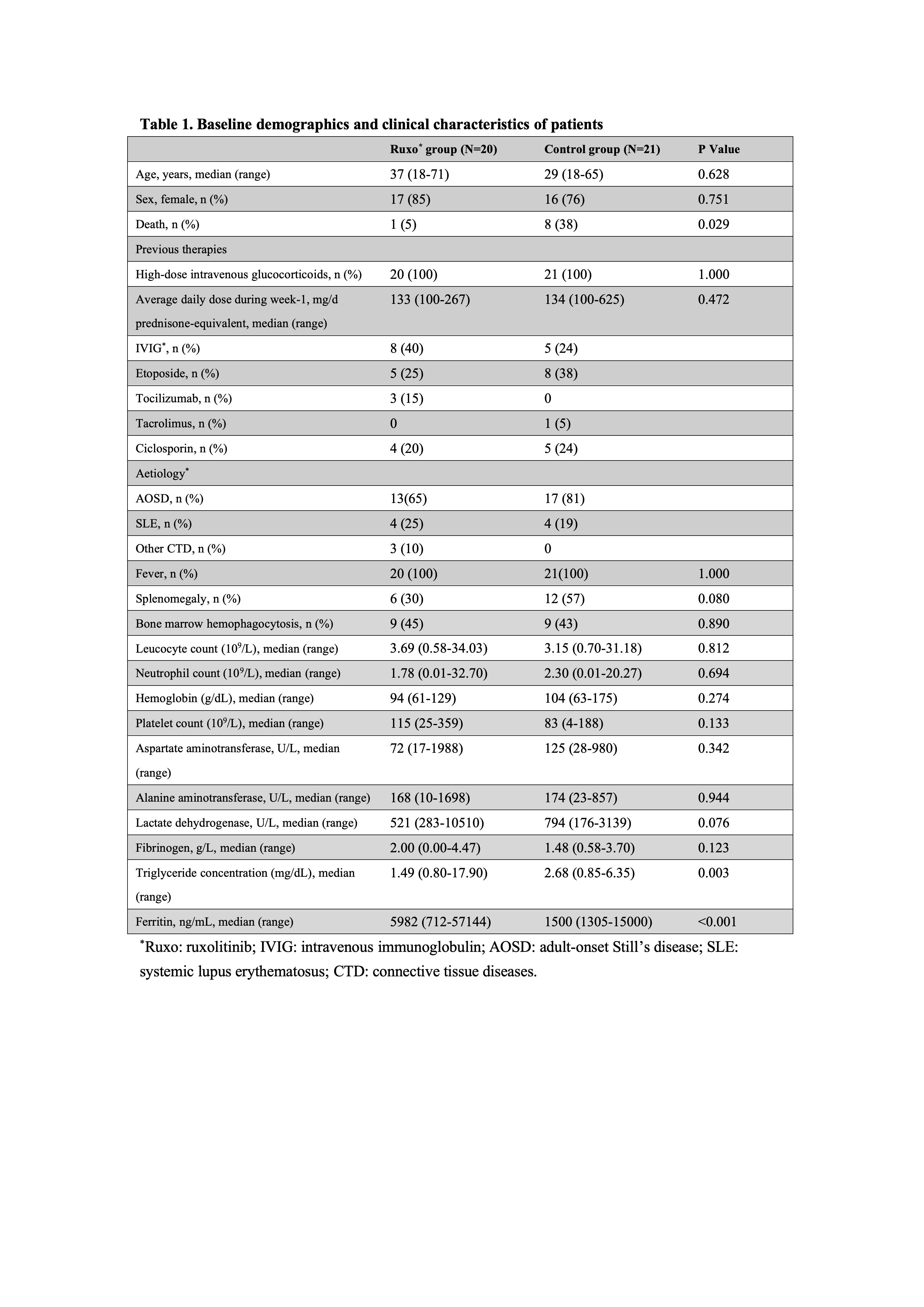 Table 1. Baseline demographics and clinical characteristics of patients
Table 1. Baseline demographics and clinical characteristics of patients
.jpg) Figure 1. Survival curve of the ruxolitinib group and the historical control group.
Figure 1. Survival curve of the ruxolitinib group and the historical control group.
.jpg) Figure 2. The response of patients with refractory CTD-MAS during the ruxolitinib treatment.
Figure 2. The response of patients with refractory CTD-MAS during the ruxolitinib treatment.
*CR: complete remission; PR: partial remission; NR: no response.
To cite this abstract in AMA style:
Li J, Wang R, Chen J, xu A, Fu Y, Lin Y, Wang X, Ye S, Du F, Fu Q. Efficacy and Safety of Ruxolitinib in Adult Patients With Refractory Connective Tissue Disease-Associated Macrophage Activation Syndrome [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-ruxolitinib-in-adult-patients-with-refractory-connective-tissue-disease-associated-macrophage-activation-syndrome/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-ruxolitinib-in-adult-patients-with-refractory-connective-tissue-disease-associated-macrophage-activation-syndrome/
