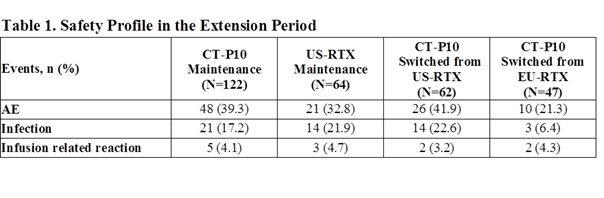Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Similarity of pharmacokinetic, efficacy and safety between CT-P10 and reference rituximab (RTX) were shown in the phase 3 randomized controlled trial (NCT02149121) up to 48 weeks in RA patients1,2,3. This is to investigate efficacy and safety of CT-P10 when used for long term and after switching from RTX in the extension phase of above study.
Methods: Patients who completed up to 48 weeks (Main Period), entered into the Extension Period. Patients who had received CT-P10 or EU-RTX in the Main Period received CT-P10 and patients who had received US-RTX randomly assigned in a 1:1 ratio to receive CT-P10 or US-RTX at Extension Weeks 0 and 2. Efficacy, pharmacodynamics (PD), safety and immunogenicity were evaluated for 24 weeks.
Results: A total of 295 patients (122 CT-P10 Maintenance, 64 US-RTX Maintenance, 62 CT-P10 Switched from US-RTX and 47 CT-P10 Switched from EU-RTX groups) were treated in the Extension Period.
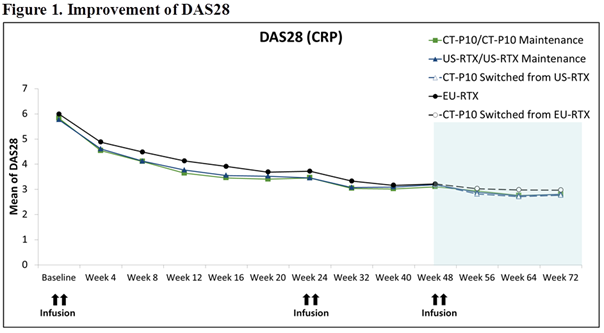
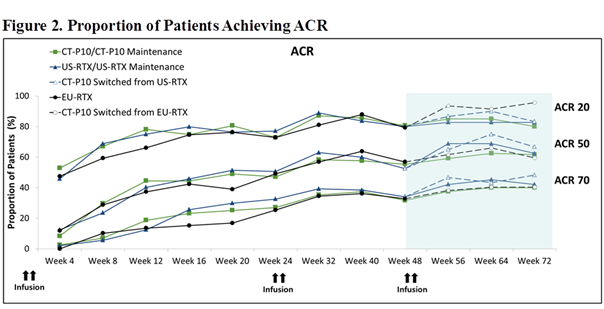
The mean changes of DAS28 from baseline of Main Period and ACR response rate were comparable among the groups (Figures 1 and 2).
B-cell depletion after the 1st infusion was comparable and maintained until Extension Week 24 in all groups.
No remarkable changes in immunogenicity profile were observed following the drug switch. Two patients (1 patient each in the US-RTX Maintenance and CT-P10 Switched from US-RTX groups) had anti-drug antibody newly developed after Extension Week 0 infusion.
The safety profiles were also comparable among the groups (Table 1). All infusion related reactions were grade 1 or 2 intensity. Most frequent infections were upper respiratory and urinary tract infections. No malignancy, progressive multifocal leukoencephalopathy or death were reported.
Conclusion: Long term effectiveness and tolerability were achieved over 72-week treatment of CT-P10. The switched groups from RTX to CT-P10 were comparable to CT-P10 or US-RTX Maintenance groups in the efficacy, PD and safety profiles including immunogenicity.
Reference
1. Suh CH, et al. Arthritis Rheum 2016;68(Suppl 10): 2037-9
2. Yoo DH, et al. Arthritis Rheum 2016;68(Suppl 10): 2039-41
3. Suh CH, et al. Ann Rheum Dis 2017; 76(Suppl 2): 824-5
To cite this abstract in AMA style:
Shim SC, Bozic Majstorovic L, Berrocal Kasay A, Chalouhi El-Khouri E, Irazoque-Palazuelos F, Cons Molina F, Medina-Rodriguez FG, Miranda P, Shesternya P, Chavez Corrales J, Wiland P, Jeka S, Garmish O, Hrycaj P, Fomina N, Park W, Suh CH, Lee SJ, Lee SY, Yoo DH. Efficacy and Safety of Rituximab Biosimilar, CT-P10, after a Single Switch from Innovator Rituximabs in Patients with Rheumatoid Arthritis: Results from Phase 3 Randomized Controlled Trial over 72 Weeks [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-rituximab-biosimilar-ct-p10-after-a-single-switch-from-innovator-rituximabs-in-patients-with-rheumatoid-arthritis-results-from-phase-3-randomized-controlled-trial-over-72-wee/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-rituximab-biosimilar-ct-p10-after-a-single-switch-from-innovator-rituximabs-in-patients-with-rheumatoid-arthritis-results-from-phase-3-randomized-controlled-trial-over-72-wee/