Session Information
Date: Saturday, November 6, 2021
Title: Miscellaneous Rheumatic & Inflammatory Diseases Poster I (0183–0209)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Risankizumab (RZB) is a humanized immunoglobulin G1 monoclonal antibody that specifically inhibits interleukin 23 by binding to its p19 subunit. RZB is being investigated as a treatment for adults with psoriatic arthritis (PsA).
Methods: KEEPsAKE 2, a double-blind, phase 3 trial, evaluated the safety and efficacy of RZB vs. placebo (PBO) for the treatment of active PsA in patients that had previous inadequate response or intolerance to conventional synthetic disease modifying antirheumatic drug (csDMARD-IR) or 1 or 2 biologic therapies (Bio-IR). The KEEPsAKE 2 trial enrolled adults with a diagnosis of active PsA, active plaque psoriasis or nail psoriasis, ≥ 5 swollen joints, and ≥ 5 tender joints.
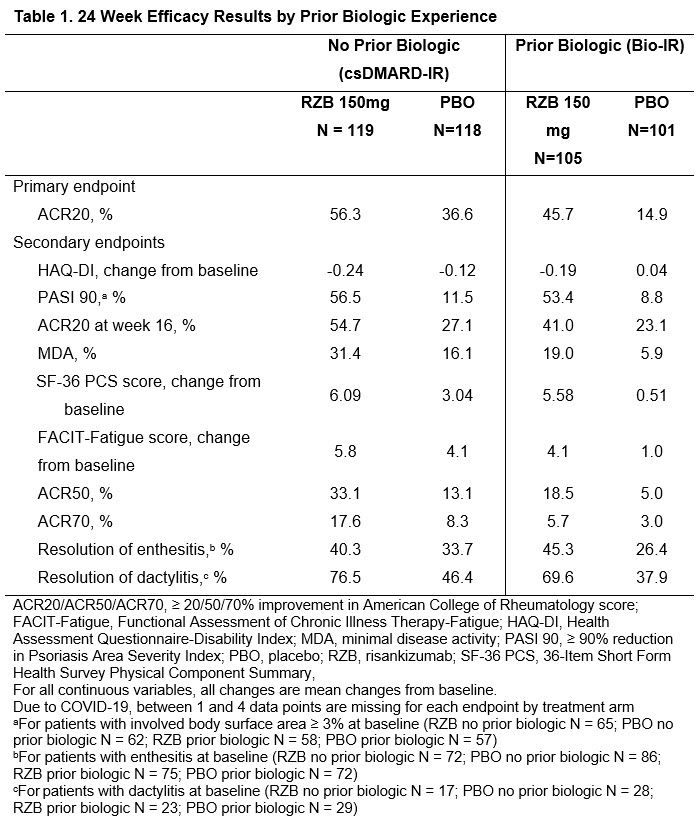
Patients were randomized (1:1) to receive blinded subcutaneous RZB 150 mg or PBO at weeks 0, 4, and 16. The primary endpoint was the proportion of patients achieving 20% improvement in the American College of Rheumatology score (ACR20) at week 24. Table 1 shows primary and secondary endpoints by prior biologic therapy utilization (0 or ≥ 1). Efficacy was assessed using non-responder imputation incorporating multiple imputations to handle missing data due to COVID-19 (NRI-C) for categorical data and Mixed-Effect Model Repeated Measurement (MMRM) analysis for continuous data. Safety was assessed throughout the study. Results reported here are from the 24-week double-blind period; the open-label period with all patients receiving RZB is ongoing.
Results: A total of 443 patients completed the KEEPsAKE 2 analysis at 24 weeks. Overall, demographics and baseline disease characteristics were similar between the patients receiving RZB and patients receiving PBO; 237 patients were csDMARD-IR (119 RZB and 118 PBO) and 206 patients were Bio-IR (105 RZB and 101 PBO). A numerically higher proportion of patients receiving RZB achieved improvement in disease severity measures compared to those receiving PBO, regardless of prior biologic experience (Table 1). Patients receiving RZB reported numerically greater improvements in patient-reported outcomes compared to those receiving PBO, regardless of prior biologic experience (Table 1). RZB was well tolerated in patients compared to PBO, and no new safety signals were observed.
Conclusion: RZB treatment resulted in improvements in signs and symptoms of PsA compared with PBO in both csDMARD-IR and Bio-IR patients and was well tolerated with no new safety signals.
To cite this abstract in AMA style:
Lidar M, Aelion J, Tarr G, Papp K, Barcomb L, Soliman A, Lu W, Eldred A, Ostor A. Efficacy and Safety of Risankizumab for Active Psoriatic Arthritis: 24-Week Results from the Phase 3, Randomized, Double-blind Clinical Trial for CsDMARD-IR and Bio-IR Patients [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-risankizumab-for-active-psoriatic-arthritis-24-week-results-from-the-phase-3-randomized-double-blind-clinical-trial-for-csdmard-ir-and-bio-ir-patients/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-risankizumab-for-active-psoriatic-arthritis-24-week-results-from-the-phase-3-randomized-double-blind-clinical-trial-for-csdmard-ir-and-bio-ir-patients/