Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: PsA is a chronic, systemic inflammatory disease with a variety of clinical manifestations including arthritis, enthesitis, and dactylitis and is associated with skin and nail psoriasis. Risankizumab (RZB) is a humanized immunoglobulin G1 monoclonal antibody that specifically inhibits interleukin 23 by binding to its p19 subunit and is approved to treat adults with active PsA.
Methods: KEEPsAKE 1 and 2 are ongoing phase 3 trials evaluating the efficacy and safety of RZB versus placebo (PBO) for the treatment of active PsA. Both trials enrolled adults with active PsA (symptom onset ≥ 6 months before screening, meeting Classification Criteria for PsA [CASPAR], and ≥ 5 tender and ≥ 5 swollen joints) and plaque or nail psoriasis. Patients enrolled in KEEPsAKE 1 had an inadequate response or intolerance to ≥ 1 conventional synthetic disease modifying antirheumatic drug (csDMARD-IR). KEEPsAKE 2 enrolled csDMARD-IR and/or patients with an inadequate response or intolerance to 1 or 2 biologic therapies (Bio-IR). Patients were randomized 1:1 to receive blinded subcutaneous RZB 150 mg or PBO at weeks 0, 4, and 16. Starting at week 24, all patients received open-label RZB 150 mg every 12 weeks. Efficacy and safety results reported here are analyzed in patients who received ≥ 1 dose of study drug through week 100. Statistical reporting and imputation methods are labeled and defined with their respective efficacy outputs. Treatment-emergent adverse events (TEAEs) were summarized using exposure-adjusted event rates (EAERs, events/100 patient-years [PYs]).
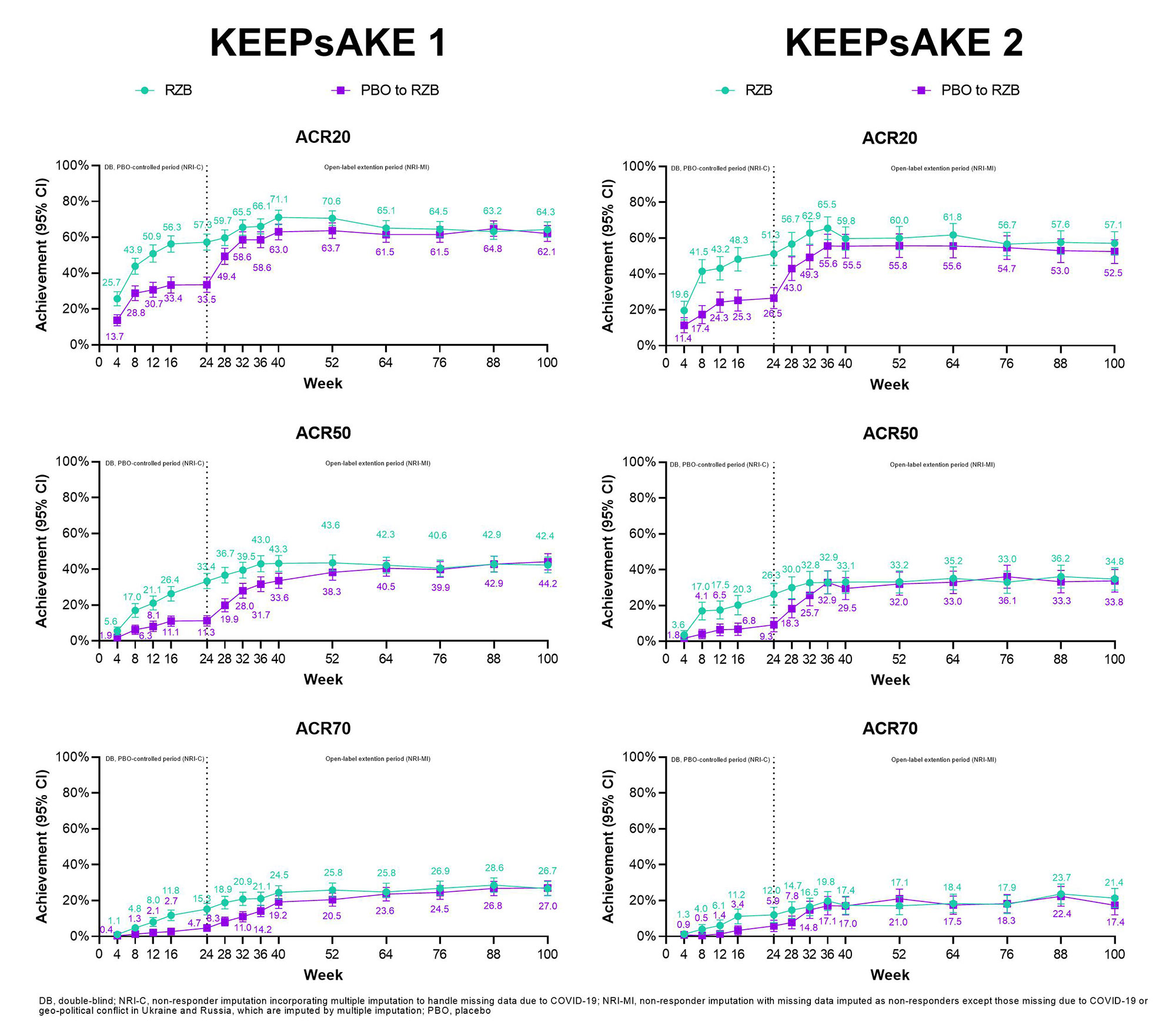
Results: At week 100, patients in KEEPsSAKE 1 (RZB N=483; PBO/RZB N=481) and KEEPsAKE 2 (RZB N=224; PBO/RZB N=219) had similar results to those reported at week 52 (Table 1). Achievement of ACR20, 50, and 70 overtime results for both studies are presented in Figure 1. In KEEPsAKE 1, ACR20 at week 100 was achieved by 64.3% and 62.1% of RZB and PBO/RZB patients, respectively. In KEEPsAKE 2, 57.1% of RZB and 52.5% of PBO/RZB patients achieved ACR20 at week 100. PASI 90 achievement levels in KEEPsAKE 1 were 71.3% for RZB patients and 67.8% for PBO/RZB patients. In KEEPsAKE 2, 67.5% of RZB and 61.3% of PBO/RZB patients achieved PASI 90. Patients also maintained HAQ-DI scores in both KEEPsAKE 1 (RZB -0.41, PBO/RZB -0.36) and KEEPsAKE 2 (RZB -0.26, PBO/RZB -0.31). For patients with nail psoriasis at baseline both PGA-F scores (KEEPsAKE 1 RZB -1.4, PBO/RZB -1.3) and mNAPSI scores (KEEPsAKE 1 RZB -11.33, PBO/RZB -13.54) were maintained. Resolution of enthesitis for patients with enthesitis at baseline was seen in 60.6% of RZB and 62.1% of PBO/RZB patients in KEEPsAKE 1 and 51.7% of RZB and 53.2% of PBO/RZB patients in KEEPsAKE 2. Resolution of dactylitis for patients with dactylitis at baseline was seen in 75.4% of RZB and 77.9% of PBO/RZB KEEPsAKE 1 patients and 77.5% of RZB and 68.4% of RZB/PBO patients in KEEPsAKE 2. As of the week 100 cut-off, the EAERs of any TEAE in KEEPsAKE 1 patients was 130.1/100PY and 180.5/100PY for KEEPsAKE 2 patients (Table 2).
Conclusion: Long term treatment with RZB provides durable efficacy response in PsA through 100 weeks. RZB was generally well tolerated, and there were new safety signals.
To cite this abstract in AMA style:
Erik L, Papp K, White D, Asnal C, Lu W, Soliman A, Padilla B, Chen M, Ostor A. Efficacy and Safety of Risankizumab for Active Psoriatic Arthritis: 100-Week Results from the KEEPsAKE 1 and KEEPsAKE 2 Trials [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-risankizumab-for-active-psoriatic-arthritis-100-week-results-from-the-keepsake-1-and-keepsake-2-trials/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-risankizumab-for-active-psoriatic-arthritis-100-week-results-from-the-keepsake-1-and-keepsake-2-trials/